Topomimetics of amphipathic beta-sheet and helix-forming bactericidal peptides neutralize lipopolysaccharide endotoxins
- PMID: 17181157
- PMCID: PMC4242098
- DOI: 10.1021/jm0610447
Topomimetics of amphipathic beta-sheet and helix-forming bactericidal peptides neutralize lipopolysaccharide endotoxins
Abstract
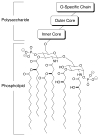
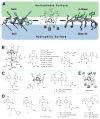
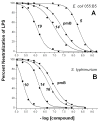
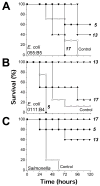
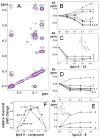
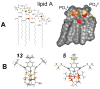
Release of lipopolysaccharide (LPS) endotoxin from Gram negative bacterial membranes triggers macrophages to produce large quantities of cytokines that can lead to septic shock and eventual death. Agents that bind to and neutralize LPS may provide a means to clinically prevent septic shock upon bacterial infection. Previously, we reported the design of antibacterial helix peptide SC4 and beta-sheet-forming betapep peptides that neutralize LPS in vitro. We hypothesized that the ability of these and other such peptides to neutralize LPS rested in the common denominator of positively charged amphipathic structure. Here, we describe the design and synthesis of nonpeptide, calixarene-based helix/sheet topomimetics that mimic the folded conformations of these peptides in their molecular dimensions, amphipathic surface topology, and compositional properties. From a small library of topomimetics, we identified several compounds that neutralize LPS in the 10-8 M range, making them as effective as bactericidal/permeability increasing protein and polymyxin B. In an endotoxemia mouse model, three of the most in vitro effective topomimetics are shown to be at least partially protective against challenges of LPS from different bacterial species. NMR studies provide mechanistic insight by suggesting the site of molecular interaction between topomimetics and the lipid A component of LPS, with binding being mediated by electrostatic and hydrophobic interactions. This research contributes to the development of pharmaceutical agents against endotoxemia and septic shock.
Figures

Similar articles
-
Designed beta-sheet-forming peptide 33mers with potent human bactericidal/permeability increasing protein-like bactericidal and endotoxin neutralizing activities.Biochim Biophys Acta. 1998 Sep 16;1425(1):81-92. doi: 10.1016/s0304-4165(98)00053-1. Biochim Biophys Acta. 1998. PMID: 9813253
-
A synthetic peptide derived from bactericidal/permeability-increasing protein neutralizes endotoxin in vitro and in vivo.Int Immunopharmacol. 2004 Apr;4(4):527-37. doi: 10.1016/j.intimp.2004.02.004. Int Immunopharmacol. 2004. PMID: 15099530
-
Anti-Endotoxin 9-Meric Peptide with Therapeutic Potential for the Treatment of Endotoxemia.J Microbiol Biotechnol. 2021 Jan 28;31(1):25-32. doi: 10.4014/jmb.2011.11011. J Microbiol Biotechnol. 2021. PMID: 33263333 Free PMC article.
-
Antimicrobial peptide rBPI21: a translational overview from bench to clinical studies.Curr Protein Pept Sci. 2012 Nov;13(7):611-9. doi: 10.2174/138920312804142101. Curr Protein Pept Sci. 2012. PMID: 23116442 Review.
-
The bactericidal/permeability increasing protein of neutrophils is a potent antibacterial and anti-endotoxin agent in vitro and in vivo.Prog Clin Biol Res. 1994;388:41-51. Prog Clin Biol Res. 1994. PMID: 7831373 Review.
Cited by
-
Polycationic calixarene PTX013, a potent cytotoxic agent against tumors and drug resistant cancer.Invest New Drugs. 2013 Oct;31(5):1142-50. doi: 10.1007/s10637-013-9932-0. Epub 2013 Feb 8. Invest New Drugs. 2013. PMID: 23392775 Free PMC article.
-
Calix[4]arene Based Highly Efficient Fluorescent Sensor for Au(3+) and I(.).J Fluoresc. 2015 Sep;25(5):1507-15. doi: 10.1007/s10895-015-1642-x. Epub 2015 Aug 20. J Fluoresc. 2015. PMID: 26289105
-
Synthesis of Norbornane Bisether Antibiotics via Silver-mediated Alkylation.RSC Adv. 2015;5:28582-28596. doi: 10.1039/C5RA03321G. RSC Adv. 2015. PMID: 26251697 Free PMC article.
-
Usefulness of ELISA Methods for Assessing LPS Interactions with Proteins and Peptides.PLoS One. 2016 Jun 1;11(6):e0156530. doi: 10.1371/journal.pone.0156530. eCollection 2016. PLoS One. 2016. PMID: 27249227 Free PMC article.
-
What Can Pleiotropic Proteins in Innate Immunity Teach Us about Bioconjugation and Molecular Design?Bioconjug Chem. 2018 Jul 18;29(7):2127-2139. doi: 10.1021/acs.bioconjchem.8b00176. Epub 2018 Jun 14. Bioconjug Chem. 2018. PMID: 29771496 Free PMC article. Review.
References
-
- Carcillo JA, Cunnion RE. Septic shock. Crit Care Clin. 1997;13:553–574. - PubMed
-
- Gagliardi AR, Hennig B, Collins DC. Antiestrogens inhibit endothelial cell growth stimulated by angiogenic growth factors. Anticancer Res. 1996;16:1101–1106. - PubMed
-
- Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–787. - PubMed
-
- Ulevitch RJ. Recognition of bacterial endotoxins by receptor-dependent mechanisms. Adv Immunol. 1993;55:267–289. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

