Human antimicrobial peptide LL-37 is present in atherosclerotic plaques and induces death of vascular smooth muscle cells: a laboratory study
- PMID: 17181861
- PMCID: PMC1764755
- DOI: 10.1186/1471-2261-6-49
Human antimicrobial peptide LL-37 is present in atherosclerotic plaques and induces death of vascular smooth muscle cells: a laboratory study
Abstract
Background: Death of smooth muscle cells in the atherosclerotic plaques makes the plaques more prone to rupture, which can initiate an acute ischemic event. The development of atherosclerosis includes the migration of immune cells e.g. monocytes/macrophages and T lymphocytes into the lesions. Immune cells can release antimicrobial peptides. One of these, human cathelicidin antimicrobial peptide hCAP-18, is cleaved by proteinase 3 generating a 4.5 kDa C-terminal fragment named LL-37, which has been shown to be cytotoxic. The aim of the study was to explore a potential role of LL-37 in the pathophysiology of atherosclerosis.
Methods: We investigated the presence of LL-37 in human atherosclerotic lesions obtained at autopsy using immunohistochemistry. The direct effects of LL-37 on cultured vascular smooth muscle cells and isolated neutrophil granulocytes were investigated with morphological, biochemical and flow cytometry analysis.
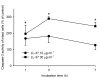
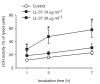
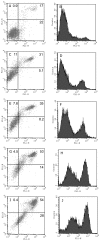
Results: The neointima of atherosclerotic plaques was found to contain LL-37-like immunoreactivity, mainly in macrophages. In cultured smooth muscle cells, LL-37 at 30 mug/ml caused cell shrinkage, membrane blebbing, nuclear condensation, DNA fragmentation and an increase in caspase-3 activity as studied by microscopy, ELISA and enzyme activity assay, respectively. Flow cytometry demonstrated that LL-37 in a subset of the cells caused a small but rapidly developing increase in membrane permeability to propidium iodide, followed by a gradual development of FITC-annexin V binding. Another cell population stained heavily with both propidium iodide and FITC-annexin V. Neutrophil granulocytes were resistant to these effects of LL-37.
Conclusion: This study shows that LL-37 is present in atherosclerotic lesions and that it induces death of vascular smooth muscle cells. In a subset of cells, the changes indicate the development of apoptosis triggered by an initial mild perturbation of plasma membrane integrity. The findings suggest a role for LL-37 as a mediator of immune cell-induced death of vascular smooth muscle cells in atherosclerosis.
Figures



Similar articles
-
Effects of human cathelicidin antimicrobial peptide LL-37 on lipopolysaccharide-induced nitric oxide release from rat aorta in vitro.Acta Anaesthesiol Scand. 2003 Feb;47(2):213-20. doi: 10.1034/j.1399-6576.2003.00045.x. Acta Anaesthesiol Scand. 2003. PMID: 12631052
-
Neutrophil secondary necrosis is induced by LL-37 derived from cathelicidin.J Leukoc Biol. 2008 Sep;84(3):780-8. doi: 10.1189/jlb.0208086. Epub 2008 Jun 4. J Leukoc Biol. 2008. PMID: 18524973 Free PMC article.
-
The Role of T Cells Reactive to the Cathelicidin Antimicrobial Peptide LL-37 in Acute Coronary Syndrome and Plaque Calcification.Front Immunol. 2020 Oct 6;11:575577. doi: 10.3389/fimmu.2020.575577. eCollection 2020. Front Immunol. 2020. PMID: 33123157 Free PMC article.
-
Estrogen actions and in situ synthesis in human vascular smooth muscle cells and their correlation with atherosclerosis.J Steroid Biochem Mol Biol. 2005 Feb;93(2-5):263-8. doi: 10.1016/j.jsbmb.2004.12.024. Epub 2005 Jan 28. J Steroid Biochem Mol Biol. 2005. PMID: 15860269 Review.
-
High-quality 3D structures shine light on antibacterial, anti-biofilm and antiviral activities of human cathelicidin LL-37 and its fragments.Biochim Biophys Acta. 2014 Sep;1838(9):2160-72. doi: 10.1016/j.bbamem.2014.01.016. Epub 2014 Jan 23. Biochim Biophys Acta. 2014. PMID: 24463069 Free PMC article. Review.
Cited by
-
Engineered Lysin-Derived Peptide as a Potent Antimicrobial for Acne Vulgaris.Antibiotics (Basel). 2025 Mar 27;14(4):344. doi: 10.3390/antibiotics14040344. Antibiotics (Basel). 2025. PMID: 40298522 Free PMC article.
-
Reactive oxygen species, apoptosis, antimicrobial peptides and human inflammatory diseases.Pharmaceuticals (Basel). 2015 Apr 2;8(2):151-75. doi: 10.3390/ph8020151. Pharmaceuticals (Basel). 2015. PMID: 25850012 Free PMC article. Review.
-
P. gingivalis in Periodontal Disease and Atherosclerosis - Scenes of Action for Antimicrobial Peptides and Complement.Front Immunol. 2015 Feb 10;6:45. doi: 10.3389/fimmu.2015.00045. eCollection 2015. Front Immunol. 2015. PMID: 25713575 Free PMC article. Review.
-
Cathelicidin: Insights into Its Impact on Metabolic Syndrome and Chronic Inflammation.Metabolites. 2024 Dec 2;14(12):672. doi: 10.3390/metabo14120672. Metabolites. 2024. PMID: 39728453 Free PMC article. Review.
-
Porphyromonas gingivalis outer membrane vesicles divert host innate immunity and promote inflammation via C4' monophosphorylated lipid A.J Immunol. 2025 May 1;214(5):1008-1021. doi: 10.1093/jimmun/vkae050. J Immunol. 2025. PMID: 40131356
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

