HIV-1 Tat interaction with Dicer: requirement for RNA
- PMID: 17181864
- PMCID: PMC1764028
- DOI: 10.1186/1742-4690-3-95
HIV-1 Tat interaction with Dicer: requirement for RNA
Abstract
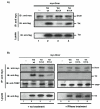
Dicer is an RNase III which processes two classes of cellular small RNAs: the microRNAs (miRNA) and short interfering RNAs (siRNA). Previously, we observed that over-expressed HIV-1 Tat protein can suppress the processing of small RNAs inside cells. Here, we have investigated the requirements for Tat interaction with Dicer. We report that Tat-Dicer interaction depends on RNA, requires the helicase domain of Dicer, and is independent of Tat's transactivation domain.
Figures


Similar articles
-
The role of PACT in the RNA silencing pathway.EMBO J. 2006 Feb 8;25(3):522-32. doi: 10.1038/sj.emboj.7600942. Epub 2006 Jan 19. EMBO J. 2006. PMID: 16424907 Free PMC article.
-
Evidence that HIV-1 encodes an siRNA and a suppressor of RNA silencing.Immunity. 2005 May;22(5):607-19. doi: 10.1016/j.immuni.2005.03.010. Immunity. 2005. PMID: 15894278
-
Site-specific cleavage of the transactivation response site of human immunodeficiency virus RNA with a tat-based chemical nuclease.Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3526-30. doi: 10.1073/pnas.89.8.3526. Proc Natl Acad Sci U S A. 1992. PMID: 1565648 Free PMC article.
-
The HIV-1 Tat transactivator protein: a therapeutic target?IUBMB Life. 2003 Dec;55(12):669-80. doi: 10.1080/15216540310001643440. IUBMB Life. 2003. PMID: 14769003 Review.
-
Biochemical and functional interactions between HIV-1 Tat protein and TAR RNA.Arch Biochem Biophys. 1999 May 15;365(2):175-85. doi: 10.1006/abbi.1999.1206. Arch Biochem Biophys. 1999. PMID: 10328810 Review.
Cited by
-
The Diverse Roles of microRNAs at the Host⁻Virus Interface.Viruses. 2018 Aug 19;10(8):440. doi: 10.3390/v10080440. Viruses. 2018. PMID: 30126238 Free PMC article. Review.
-
Robust RNAi enhancement via human Argonaute-2 overexpression from plasmids, viral vectors and cell lines.Nucleic Acids Res. 2013 Nov;41(21):e199. doi: 10.1093/nar/gkt836. Epub 2013 Sep 17. Nucleic Acids Res. 2013. PMID: 24049077 Free PMC article.
-
The microRNA miR-29a is associated with human immunodeficiency virus latency.Retrovirology. 2014 Dec 9;11:108. doi: 10.1186/s12977-014-0108-6. Retrovirology. 2014. PMID: 25486977 Free PMC article.
-
Tat is a multifunctional viral protein that modulates cellular gene expression and functions.Oncotarget. 2017 Apr 18;8(16):27569-27581. doi: 10.18632/oncotarget.15174. Oncotarget. 2017. PMID: 28187438 Free PMC article. Review.
-
Defining the molecular mechanisms of HIV-1 Tat secretion: PtdIns(4,5)P2 at the epicenter.Traffic. 2018 Apr 30:10.1111/tra.12578. doi: 10.1111/tra.12578. Online ahead of print. Traffic. 2018. PMID: 29708629 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

