Methylmercury induces oxidative injury, alterations in permeability and glutamine transport in cultured astrocytes
- PMID: 17182013
- PMCID: PMC1847599
- DOI: 10.1016/j.brainres.2006.10.070
Methylmercury induces oxidative injury, alterations in permeability and glutamine transport in cultured astrocytes
Abstract
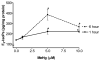
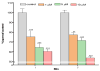
The neurotoxicity of high levels of methylmercury (MeHg) is well established both in humans and experimental animals. Astrocytes accumulate MeHg and play a prominent role in mediating MeHg toxicity in the central nervous system (CNS). Although the precise mechanisms of MeHg neurotoxicity are ill-defined, oxidative stress and altered mitochondrial and cell membrane permeability appear to be critical factors in its pathogenesis. The present study examined the effects of MeHg treatment on oxidative injury, mitochondrial inner membrane potential, glutamine uptake and expression of glutamine transporters in primary astrocyte cultures. MeHg caused a significant increase in F(2)-isoprostanes (F(2)-IsoPs), lipid peroxidation biomarkers of oxidative damage, in astrocyte cultures treated with 5 or 10 microM MeHg for 1 or 6 h. Consistent with this observation, MeHg induced a concentration-dependant reduction in the inner mitochondrial membrane potential (DeltaPsi(m)), as assessed by the potentiometric dye, tetramethylrhodamine ethyl ester (TMRE). Our results demonstrate that DeltaPsi(m) is a very sensitive endpoint for MeHg toxicity, since significant reductions were observed after only 1 h exposure to concentrations of MeHg as low as 1 microM. MeHg pretreatment (1, 5 and 10 microM) for 30 min also inhibited the net uptake of glutamine ((3)H-glutamine) measured at 1 min and 5 min. Expression of the mRNA coding the glutamine transporters, SNAT3/SN1 and ASCT2, was inhibited only at the highest (10 microM) MeHg concentration, suggesting that the reduction in glutamine uptake observed after 30 min treatment with lower concentrations of MeHg (1 and 5 microM) was not due to inhibition of transcription. Taken together, these studies demonstrate that MeHg exposure is associated with increased mitochondrial membrane permeability, alterations in glutamine/glutamate cycling, increased ROS formation and consequent oxidative injury. Ultimately, MeHg initiates multiple additive or synergistic disruptive mechanisms that lead to cellular dysfunction and cell death.
Figures






References
-
- Ali SF, LeBel CP, Bondy SC. Reactive oxygen species formation as a biomarker of methylmercury and trimethyltin neurotoxicity. Neurotoxocology. 1992;13:637 – 648. - PubMed
-
- Allen JW, El-Oqayli H, Aschner M, Syversen T, Sonnewald U. Methylmercury has a selective effect on mitochondria in cultured astrocytes in the presence of [U-13C]glutamate. Brain Res. 2001;908:149 –154. - PubMed
-
- Aschner M, Du YL, Gannon M, Kimelberg HK. Methylmercury-induced alterations in excitatory amino acid transport in rat primary astrocyte cultures. Brain Res. 1993;602:181 – 186. - PubMed
-
- Aschner M, Mullaney KJ, Wagoner D, Lash LH, Kimelberg HK. Intracellular glutathione (GSH) levels modulate mercuric chloride (MC)- and methylmercuric chloride (MeHgCl)-induced amino acid release from neonatal rat primary astrocyte cultures. Brain Res. 1994;664:133 – 140. - PubMed
-
- Aschner M. Astrocytes as modulators of mercury-induced neurotoxicity. Neurotoxicology. 1996;17:663 – 669. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

