Interaction of rotavirus polymerase VP1 with nonstructural protein NSP5 is stronger than that with NSP2
- PMID: 17182692
- PMCID: PMC1865955
- DOI: 10.1128/JVI.01494-06
Interaction of rotavirus polymerase VP1 with nonstructural protein NSP5 is stronger than that with NSP2
Abstract
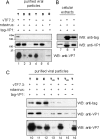
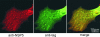
Rotavirus morphogenesis starts in intracellular inclusion bodies called viroplasms. RNA replication and packaging are mediated by several viral proteins, of which VP1, the RNA-dependent RNA polymerase, and VP2, the core scaffolding protein, were shown to be sufficient to provide replicase activity in vitro. In vivo, however, viral replication complexes also contain the nonstructural proteins NSP2 and NSP5, which were shown to be essential for replication, to interact with each other, and to form viroplasm-like structures (VLS) when coexpressed in uninfected cells. In order to gain a better understanding of the intermediates formed during viral replication, this work focused on the interactions of NSP5 with VP1, VP2, and NSP2. We demonstrated a strong interaction of VP1 with NSP5 but only a weak one with NSP2 in cotransfected cells in the absence of other viral proteins or viral RNA. By contrast, we failed to coimmunoprecipitate VP2 with anti-NSP5 antibodies or NSP5 with anti-VP2 antibodies. We constructed a tagged form of VP1, which was found to colocalize in viroplasms and in VLS formed by NSP5 and NSP2. The tagged VP1 was able to replace VP1 structurally by being incorporated into progeny viral particles. When applying anti-tag-VP1 or anti-NSP5 antibodies, coimmunoprecipitation of tagged VP1 with NSP5 was found. Using deletion mutants of NSP5 or different fragments of NSP5 fused to enhanced green fluorescent protein, we identified the 48 C-terminal amino acids as the region essential for interaction with VP1.
Figures


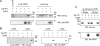




References
-
- Afrikanova, I., E. Fabbretti, M. C. Miozzo, and O. R. Burrone. 1998. Rotavirus NSP5 phosphorylation is up-regulated by interaction with NSP2. J. Gen. Virol. 79:2679-2686. - PubMed
-
- Afrikanova, I., M. C. Miozzo, S. Giambiagi, and O. Burrone. 1996. Phosphorylation generates different forms of rotavirus NSP5. J. Gen. Virol. 77:2059-2065. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

