Maternal serotonin is crucial for murine embryonic development
- PMID: 17182745
- PMCID: PMC1713169
- DOI: 10.1073/pnas.0606722104
Maternal serotonin is crucial for murine embryonic development
Abstract
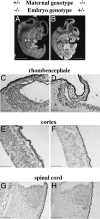
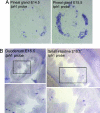
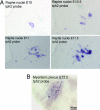
The early appearance of serotonin and its receptors during prenatal development, together with the many effects serotonin exerts during CNS morphogenesis, strongly suggest that serotonin influences the development and maturation of the mammalian brain before it becomes a neuromodulator/neurotransmitter. Sites of early serotonin biosynthesis, however, have not been detected in mouse embryos or extraembryonic structures, suggesting that the main source of serotonin could be of maternal origin. This hypothesis was tested by using knockout mice lacking the tph1 gene, which is responsible for the synthesis of peripheral serotonin. Genetic crosses were performed to compare the phenotype of pups born from homozygous and heterozygous mothers. Observations provide the first clear evidence that (i) maternal serotonin is involved in the control of morphogenesis during developmental stages that precede the appearance of serotonergic neurons and (ii) serotonin is critical for normal murine development. Most strikingly, the phenotype of tph1-/- embryos depends more on the maternal genotype than on that of the concepti. Consideration of the maternal genotype may thus help to clarify the influence of other genes in complex diseases, such as mental illness.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Moiseiwitsch JR. Crit Rev Oral Biol Med. 2000;11:230–239. - PubMed
-
- Nguyen L, Rigo JM, Rocher V, Belachew S, Malgrange B, Rogister B, Leprince P, Moonen G. Cell Tissue Res. 2001;305:187–202. - PubMed
-
- Levin M, Buznikov GA, Lauder JM. Dev Neurosci. 2006;28:171–185. - PubMed
-
- Wallace JA. J Comp Neurol. 1985;236:443–453. - PubMed
-
- Choi DS, Ward SJ, Messaddeq N, Launay J-M, Maroteaux L. Development (Cambridge, UK) 1997;124:1745–1755. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

