Mitogen-activated protein kinase kinase 1-dependent Golgi unlinking occurs in G2 phase and promotes the G2/M cell cycle transition
- PMID: 17182854
- PMCID: PMC1783781
- DOI: 10.1091/mbc.e06-06-0530
Mitogen-activated protein kinase kinase 1-dependent Golgi unlinking occurs in G2 phase and promotes the G2/M cell cycle transition
Abstract
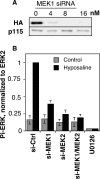
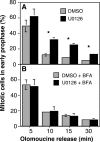
Two controversies have emerged regarding the signaling pathways that regulate Golgi disassembly at the G(2)/M cell cycle transition. The first controversy concerns the role of mitogen-activated protein kinase activator mitogen-activated protein kinase kinase (MEK)1, and the second controversy concerns the participation of Golgi structure in a novel cell cycle "checkpoint." A potential simultaneous resolution is suggested by the hypothesis that MEK1 triggers Golgi unlinking in late G(2) to control G(2)/M kinetics. Here, we show that inhibition of MEK1 by RNA interference or by using the MEK1/2-specific inhibitor U0126 delayed the passage of synchronized HeLa cells into M phase. The MEK1 requirement for normal mitotic entry was abrogated if Golgi proteins were dispersed before M phase by treatment of cells with brefeldin A or if GRASP65, which links Golgi stacks into a ribbon network, was depleted. Imaging revealed that unlinking of the Golgi apparatus begins before M phase, is independent of cyclin-dependent kinase 1 activation, and requires MEK signaling. Furthermore, expression of the GRASP family member GRASP55 after alanine substitution of its MEK1-dependent mitotic phosphorylation sites inhibited both late G(2) Golgi unlinking and the G(2)/M transition. Thus, MEK1 plays an in vivo role in Golgi reorganization, which regulates cell cycle progression.
Figures






Similar articles
-
GRASP55 regulates Golgi ribbon formation.Mol Biol Cell. 2008 Jul;19(7):2696-707. doi: 10.1091/mbc.e07-11-1200. Epub 2008 Apr 23. Mol Biol Cell. 2008. PMID: 18434598 Free PMC article.
-
MEK1 inactivates Myt1 to regulate Golgi membrane fragmentation and mitotic entry in mammalian cells.EMBO J. 2013 Jan 9;32(1):72-85. doi: 10.1038/emboj.2012.329. Epub 2012 Dec 14. EMBO J. 2013. PMID: 23241949 Free PMC article.
-
JNK2 controls fragmentation of the Golgi complex and the G2/M transition through phosphorylation of GRASP65.J Cell Sci. 2015 Jun 15;128(12):2249-60. doi: 10.1242/jcs.164871. Epub 2015 May 6. J Cell Sci. 2015. PMID: 25948586
-
Golgi ribbon unlinking: an organelle-based G2/M checkpoint.Cell Cycle. 2007 Nov 15;6(22):2723-9. doi: 10.4161/cc.6.22.4896. Epub 2007 Aug 14. Cell Cycle. 2007. PMID: 17986866 Review.
-
Golgi complex fragmentation in G2/M transition: An organelle-based cell-cycle checkpoint.IUBMB Life. 2012 Aug;64(8):661-70. doi: 10.1002/iub.1054. Epub 2012 Jun 23. IUBMB Life. 2012. PMID: 22730233 Review.
Cited by
-
PKD controls mitotic Golgi complex fragmentation through a Raf-MEK1 pathway.Mol Biol Cell. 2013 Feb;24(3):222-33. doi: 10.1091/mbc.E12-03-0198. Epub 2012 Dec 14. Mol Biol Cell. 2013. PMID: 23242995 Free PMC article.
-
Epithelial-to-mesenchymal transition drives a pro-metastatic Golgi compaction process through scaffolding protein PAQR11.J Clin Invest. 2017 Jan 3;127(1):117-131. doi: 10.1172/JCI88736. Epub 2016 Nov 21. J Clin Invest. 2017. PMID: 27869652 Free PMC article.
-
Organelle Inheritance Control of Mitotic Entry and Progression: Implications for Tissue Homeostasis and Disease.Front Cell Dev Biol. 2019 Jul 23;7:133. doi: 10.3389/fcell.2019.00133. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31396510 Free PMC article. Review.
-
GRASP55 regulates Golgi ribbon formation.Mol Biol Cell. 2008 Jul;19(7):2696-707. doi: 10.1091/mbc.e07-11-1200. Epub 2008 Apr 23. Mol Biol Cell. 2008. PMID: 18434598 Free PMC article.
-
Signaling at the Golgi during mitosis.Methods Cell Biol. 2013;118:383-400. doi: 10.1016/B978-0-12-417164-0.00023-9. Methods Cell Biol. 2013. PMID: 24295319 Free PMC article. Review.
References
-
- Acharya U., Mallabiabarrena A., Acharya J. K., Malhotra V. Signaling via mitogen-activated protein kinase kinase (MEK1) is required for Golgi fragmentation during mitosis. Cell. 1998;92:183–192. - PubMed
-
- Besson A., Davy A., Robbins S. M., Yong V. W. Differential activation of ERKs to focal adhesions by PKC epsilon is required for PMA-induced adhesion and migration of human glioma cells. Oncogene. 2001;20:7398–7407. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

