The establishment of neuronal properties is controlled by Sox4 and Sox11
- PMID: 17182872
- PMCID: PMC1698453
- DOI: 10.1101/gad.403406
The establishment of neuronal properties is controlled by Sox4 and Sox11
Abstract
The progression of neurogenesis relies on proneural basic helix-loop-helix (bHLH) transcription factors. These factors operate in undifferentiated neural stem cells and induce cell cycle exit and the initiation of a neurogenic program. However, the transient expression of proneural bHLH proteins in neural progenitors indicates that expression of neuronal traits must rely on previously unexplored mechanisms operating downstream from proneural bHLH proteins. Here we show that the HMG-box transcription factors Sox4 and Sox11 are of critical importance, downstream from proneural bHLH proteins, for the establishment of pan-neuronal protein expression. Examination of a neuronal gene promoter reveals that Sox4 and Sox11 exert their functions as transcriptional activators. Interestingly, the capacity of Sox4 and Sox11 to induce the expression of neuronal traits is independent of mechanisms regulating the exit of neural progenitors from the cell cycle. The transcriptional repressor protein REST/NRSF has been demonstrated to block neuronal gene expression in undifferentiated neural cells. We now show that REST/NRSF restricts the expression of Sox4 and Sox11, explaining how REST/NRSF can prevent precocious expression of neuronal proteins. Together, these findings demonstrate a central regulatory role of Sox4 and Sox11 during neuronal maturation and mechanistically separate cell cycle withdrawal from the establishment of neuronal properties.
Figures
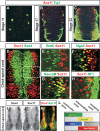


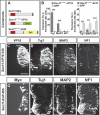


References
-
- Aarnisalo, P., Kim, C.H., Lee, J.W., Perlmann, T. Defining requirements for heterodimerization between the retinoid X receptor and the orphan nuclear receptor Nurr1. J. Biol. Chem. 2002;277:35118–35123. - PubMed
-
- Berk, A.J., Boyer, T.G., Kapanidis, A.N., Ebright, R.H., Kobayashi, N.N., Horn, P.J., Sullivan, S.M., Koop, R., Surby, M.A., Triezenberg, S.J. Mechanisms of viral activators. Cold Spring Harb. Symp. Quant. Biol. 1998;63:243–252. - PubMed
-
- Bertrand, N., Castro, D.S., Guillemot, F. Proneural genes and the specification of neural cell types. Nat. Rev. Neurosci. 2002;3:517–530. - PubMed
-
- Briscoe, J., Pierani, A., Jessell, T.M., Ericson, J. A homeodomain code specifies progenitor cell identity and neuronal fate in the ventral neural tube. Cell. 2000;101:435–445. - PubMed
-
- Bylund, M., Andersson, E., Novitch, B.G., Muhr, J. Vertebrate neurogenesis is counteracted by Sox1-3 activity. Nat. Neurosci. 2003;6:1162–1168. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
