Neural substrate of cold-seeking behavior in endotoxin shock
- PMID: 17183631
- PMCID: PMC1762328
- DOI: 10.1371/journal.pone.0000001
Neural substrate of cold-seeking behavior in endotoxin shock
Abstract
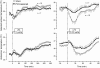
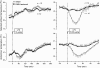
Systemic inflammation is a leading cause of hospital death. Mild systemic inflammation is accompanied by warmth-seeking behavior (and fever), whereas severe inflammation is associated with cold-seeking behavior (and hypothermia). Both behaviors are adaptive. Which brain structures mediate which behavior is unknown. The involvement of hypothalamic structures, namely, the preoptic area (POA), paraventricular nucleus (PVH), or dorsomedial nucleus (DMH), in thermoregulatory behaviors associated with endotoxin (lipopolysaccharide [LPS])-induced systemic inflammation was studied in rats. The rats were allowed to select their thermal environment by freely moving in a thermogradient apparatus. A low intravenous dose of Escherichia coli LPS (10 microg/kg) caused warmth-seeking behavior, whereas a high, shock-inducing dose (5,000 microg/kg) caused cold-seeking behavior. Bilateral electrocoagulation of the PVH or DMH, but not of the POA, prevented this cold-seeking response. Lesioning the DMH with ibotenic acid, an excitotoxin that destroys neuronal bodies but spares fibers of passage, also prevented LPS-induced cold-seeking behavior; lesioning the PVH with ibotenate did not affect it. Lesion of no structure affected cold-seeking behavior induced by heat exposure or by pharmacological stimulation of the transient receptor potential (TRP) vanilloid-1 channel ("warmth receptor"). Nor did any lesion affect warmth-seeking behavior induced by a low dose of LPS, cold exposure, or pharmacological stimulation of the TRP melastatin-8 ("cold receptor"). We conclude that LPS-induced cold-seeking response is mediated by neuronal bodies located in the DMH and neural fibers passing through the PVH. These are the first two landmarks on the map of the circuitry of cold-seeking behavior associated with endotoxin shock.
Conflict of interest statement
Figures







References
-
- Romanovsky AA. Chapter 23. Temperature regulation. In: Petersen O, editor. Lecture Notes on Human Physiology. Oxford: Blackwell; 2006. pp. 603–615. 5th ed.
-
- Roberts WW. Differential thermosensor control of thermoregulatory grooming, locomotion, and relaxed postural extension. Ann N Y Acad Sci. 1988;525:363–374. - PubMed
-
- Nagashima K, Nakai S, Tanaka M, Kanosue K. Neuronal circuitries involved in thermoregulation. Auton Neurosci. 2000;85:18–25. - PubMed
-
- Florez-Duquet M, Peloso E, Satinoff E. Fever and behavioral thermoregulation in young and old rats. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1457–R1461. - PubMed
-
- Almeida M, Steiner AA, Branco LG, Romanovsky AA. Cold-seeking behavior as a thermoregulatory strategy in systemic inflammation. Eur J Neurosci. 2006;23:3359–3367. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

