Development of a tumor-selective approach to treat metastatic cancer
- PMID: 17183650
- PMCID: PMC1762394
- DOI: 10.1371/journal.pone.0000023
Development of a tumor-selective approach to treat metastatic cancer
Abstract
Background: Patients diagnosed with metastatic cancer have almost uniformly poor prognoses. The treatments available for patients with disseminated disease are usually not curative and have side effects that limit the therapy that can be given. A treatment that is selectively toxic to tumors would maximize the beneficial effects of therapy and minimize side effects, potentially enabling effective treatment to be administered.
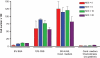
Methods and findings: We postulated that the tumor-tropic property of stem cells or progenitor cells could be exploited to selectively deliver a therapeutic gene to metastatic solid tumors, and that expression of an appropriate transgene at tumor loci might mediate cures of metastatic disease. To test this hypothesis, we injected HB1.F3.C1 cells transduced to express an enzyme that efficiently activates the anti-cancer prodrug CPT-11 intravenously into mice bearing disseminated neuroblastoma tumors. The HB1.F3.C1 cells migrated selectively to tumor sites regardless of the size or anatomical location of the tumors. Mice were then treated systemically with CPT-11, and the efficacy of treatment was monitored. Mice treated with the combination of HB1.F3.C1 cells expressing the CPT-11-activating enzyme and this prodrug produced tumor-free survival of 100% of the mice for >6 months (P<0.001 compared to control groups).
Conclusions: The novel and significant finding of this study is that it may be possible to exploit the tumor-tropic property of stem or progenitor cells to mediate effective, tumor-selective therapy for metastatic tumors, for which no tolerated curative treatments are currently available.
Conflict of interest statement
Figures







References
-
- Matthay KK, Seeger RC, Reynolds CP, Stram DO, O'Leary MC, et al. Allogeneic versus autologous purged bone marrow transplantation for neuroblastoma: a report from the Children's Cancer Group. J Clin Oncol. 1994;12:2382–2389. - PubMed
-
- Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003;3:203–216. - PubMed
-
- Evans AE, August CS, Kamani N, Bunin N, Goldwein J, et al. Bone marrow transplantation for high risk neuroblastoma at the Children's Hospital of Philadelphia: an update. Med Pediatr Oncol. 1994;23:323–327. - PubMed
-
- Ourednik V, Ourednik J, Park KI, Teng YD, Aboody KS, et al. Neural stem cells are uniquely suited for cell replacement and gene therapy in the CNS. Novartis Found Symp. 2000;231:242–262. Discussion 262–249, 302–246. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

