Triose phosphate isomerase deficiency is caused by altered dimerization--not catalytic inactivity--of the mutant enzymes
- PMID: 17183658
- PMCID: PMC1762313
- DOI: 10.1371/journal.pone.0000030
Triose phosphate isomerase deficiency is caused by altered dimerization--not catalytic inactivity--of the mutant enzymes
Abstract
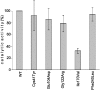
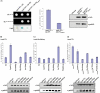
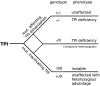
Triosephosphate isomerase (TPI) deficiency is an autosomal recessive disorder caused by various mutations in the gene encoding the key glycolytic enzyme TPI. A drastic decrease in TPI activity and an increased level of its substrate, dihydroxyacetone phosphate, have been measured in unpurified cell extracts of affected individuals. These observations allowed concluding that the different mutations in the TPI alleles result in catalytically inactive enzymes. However, despite a high occurrence of TPI null alleles within several human populations, the frequency of this disorder is exceptionally rare. In order to address this apparent discrepancy, we generated a yeast model allowing us to perform comparative in vivo analyses of the enzymatic and functional properties of the different enzyme variants. We discovered that the majority of these variants exhibit no reduced catalytic activity per se. Instead, we observed, the dimerization behavior of TPI is influenced by the particular mutations investigated, and by the use of a potential alternative translation initiation site in the TPI gene. Additionally, we demonstrated that the overexpression of the most frequent TPI variant, Glu104Asp, which displays altered dimerization features, results in diminished endogenous TPI levels in mammalian cells. Thus, our results reveal that enzyme deregulation attributable to aberrant dimerization of TPI, rather than direct catalytic inactivation of the enzyme, underlies the pathogenesis of TPI deficiency. Finally, we discovered that yeast cells expressing a TPI variant exhibiting reduced catalytic activity are more resistant against oxidative stress caused by the thiol-oxidizing reagent diamide. This observed advantage might serve to explain the high allelic frequency of TPI null alleles detected among human populations.
Conflict of interest statement
Figures





Similar articles
-
Triosephosphate isomerase deficiency: consequences of an inherited mutation at mRNA, protein and metabolic levels.Biochem J. 2005 Dec 15;392(Pt 3):675-83. doi: 10.1042/BJ20050993. Biochem J. 2005. PMID: 16086671 Free PMC article.
-
Missense variant in TPI1 (Arg189Gln) causes neurologic deficits through structural changes in the triosephosphate isomerase catalytic site and reduced enzyme levels in vivo.Biochim Biophys Acta Mol Basis Dis. 2019 Sep 1;1865(9):2257-2266. doi: 10.1016/j.bbadis.2019.05.002. Epub 2019 May 7. Biochim Biophys Acta Mol Basis Dis. 2019. PMID: 31075491 Free PMC article.
-
Distinct behavior of mutant triosephosphate isomerase in hemolysate and in isolated form: molecular basis of enzyme deficiency.Blood. 2001 Nov 15;98(10):3106-12. doi: 10.1182/blood.v98.10.3106. Blood. 2001. PMID: 11698297
-
Triosephosphate isomerase deficiency: facts and doubts.IUBMB Life. 2006 Dec;58(12):703-15. doi: 10.1080/15216540601115960. IUBMB Life. 2006. PMID: 17424909 Review.
-
Triosephosphate isomerase deficiency: new insights into an enigmatic disease.Biochim Biophys Acta. 2009 Dec;1792(12):1168-74. doi: 10.1016/j.bbadis.2009.09.012. Epub 2009 Sep 26. Biochim Biophys Acta. 2009. PMID: 19786097 Review.
Cited by
-
The difference between rare and exceptionally rare: molecular characterization of ribose 5-phosphate isomerase deficiency.J Mol Med (Berl). 2010 Sep;88(9):931-9. doi: 10.1007/s00109-010-0634-1. Epub 2010 May 25. J Mol Med (Berl). 2010. PMID: 20499043
-
Revving an Engine of Human Metabolism: Activity Enhancement of Triosephosphate Isomerase via Hemi-Phosphorylation.ACS Chem Biol. 2022 Oct 21;17(10):2769-2780. doi: 10.1021/acschembio.2c00324. Epub 2022 Aug 11. ACS Chem Biol. 2022. PMID: 35951581 Free PMC article.
-
Triosephosphate isomerase I170V alters catalytic site, enhances stability and induces pathology in a Drosophila model of TPI deficiency.Biochim Biophys Acta. 2015 Jan;1852(1):61-9. doi: 10.1016/j.bbadis.2014.10.010. Epub 2014 Oct 16. Biochim Biophys Acta. 2015. PMID: 25463631 Free PMC article.
-
Hsp70- and Hsp90-mediated proteasomal degradation underlies TPI sugarkill pathogenesis in Drosophila.Neurobiol Dis. 2010 Dec;40(3):676-83. doi: 10.1016/j.nbd.2010.08.011. Epub 2010 Aug 19. Neurobiol Dis. 2010. PMID: 20727972 Free PMC article.
-
Prediction of the functional effect of novel SLC25A13 variants using a S. cerevisiae model of AGC2 deficiency.J Inherit Metab Dis. 2013 Sep;36(5):821-30. doi: 10.1007/s10545-012-9543-5. Epub 2012 Oct 3. J Inherit Metab Dis. 2013. PMID: 23053473
References
-
- Schneider AS, Valentine WN, Hattori M, Heins HL., Jr Hereditary Hemolytic Anemia with Triosephosphate Isomerase Deficiency. N Engl J Med. 1965;272:229–235. - PubMed
-
- Schneider AS. Triosephosphate isomerase deficiency: historical perspectives and molecular aspects. Baillieres Best Pract Res Clin Haematol. 2000;13:119–140. - PubMed
-
- Schneider A, Cohen-Solal M. Hematologically important mutations: triosephosphate isomerase. Blood Cells Mol Dis. 1996;22:82–84. - PubMed
-
- Olah J, Orosz F, Keseru GM, Kovari Z, Kovacs J, et al. Triosephosphate isomerase deficiency: a neurodegenerative misfolding disease. Biochem Soc Trans. 2002;30:30–38. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

