A weakened transcriptional enhancer yields variegated gene expression
- PMID: 17183661
- PMCID: PMC1762374
- DOI: 10.1371/journal.pone.0000033
A weakened transcriptional enhancer yields variegated gene expression
Abstract
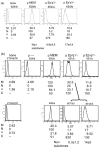
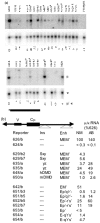
Identical genes in the same cellular environment are sometimes expressed differently. In some cases, including the immunoglobulin heavy chain (IgH) locus, this type of differential gene expression has been related to the absence of a transcriptional enhancer. To gain additional information on the role of the IgH enhancer, we examined expression driven by enhancers that were merely weakened, rather than fully deleted, using both mutations and insulators to impair enhancer activity. For this purpose we used a LoxP/Cre system to place a reporter gene at the same genomic site of a stable cell line. Whereas expression of the reporter gene was uniformly high in the presence of the normal, uninsulated enhancer and undetectable in its absence, weakened enhancers yielded variegated expression of the reporter gene; i.e., the average level of expression of the same gene differed in different clones, and expression varied significantly among cells within individual clones. These results indicate that the weakened enhancer allows the reporter gene to exist in at least two states. Subtle aspects of the variegation suggest that the IgH enhancer decreases the average duration (half-life) of the silent state. This analysis has also tested the conventional wisdom that enhancer activity is independent of distance and orientation. Thus, our analysis of mutant (truncated) forms of the IgH enhancer revealed that the 250 bp core enhancer was active in its normal position, approximately 1.4 kb 3' of the promoter, but inactive approximately 6 kb 3', indicating that the activity of the core enhancer was distance-dependent. A longer segment--the core enhancer plus approximately 1 kb of 3' flanking material, including the 3' matrix attachment region--was active, and the activity of this longer segment was orientation-dependent. Our data suggest that this 3' flank includes binding sites for at least two activators.
Conflict of interest statement
Figures





Similar articles
-
Replacement of Imu-Cmu intron by NeoR gene alters Imu germ-line expression but has no effect on V(D)J recombination.Mol Immunol. 2010 Feb;47(5):961-71. doi: 10.1016/j.molimm.2009.11.024. Epub 2009 Dec 28. Mol Immunol. 2010. PMID: 20036775
-
Analysis of hypermutation in immunoglobulin heavy chain passenger transgenes.Eur J Immunol. 1996 May;26(5):1058-62. doi: 10.1002/eji.1830260515. Eur J Immunol. 1996. PMID: 8647167
-
The Eμ enhancer region influences H chain expression and B cell fate without impacting IgVH repertoire and immune response in vivo.J Immunol. 2014 Aug 1;193(3):1171-83. doi: 10.4049/jimmunol.1302868. Epub 2014 Jun 25. J Immunol. 2014. PMID: 24965776
-
A system for precise analysis of transcription-regulating elements of immunoglobulin genes.BMC Biotechnol. 2005 Oct 4;5:27. doi: 10.1186/1472-6750-5-27. BMC Biotechnol. 2005. PMID: 16202157 Free PMC article.
-
Identification and localization of an enhancer for the human lambda L chain Ig gene complex.J Immunol. 1991 Oct 1;147(7):2354-8. J Immunol. 1991. PMID: 1918967
Cited by
-
Requirement for enhancer specificity in immunoglobulin heavy chain locus regulation.J Immunol. 2008 Jun 1;180(11):7443-50. doi: 10.4049/jimmunol.180.11.7443. J Immunol. 2008. PMID: 18490744 Free PMC article.
-
Creation and analysis of a novel chimeric promoter for the complete containment of pollen- and seed-mediated gene flow.Plant Cell Rep. 2008 Jun;27(6):995-1004. doi: 10.1007/s00299-008-0522-0. Epub 2008 Mar 4. Plant Cell Rep. 2008. PMID: 18317776
References
-
- Hume DA. Probability in transcriptional regulation and its implications for leukocyte differentiation and inducible gene expression. Blood. 2000;96:2323–2328. - PubMed
-
- Kurakin A. Self-organization vs Watchmaker: stochastic gene expression and cell differentiation. Dev Genes Evol. 2005;215:46–52. - PubMed
-
- Serizawa S, Miyamichi K, Sakano H. One neuron-one receptor rule in the mouse olfactory system. Trends Genet. 2005;20:648–653. - PubMed
-
- Guo L, Hu-Li J, Paul W. Probabilistic regulation in TH2 cells accounts for monoallelic expression of IL-4 and IL-13. Immunity. 2005;23:89–99. - PubMed
-
- Martin DIK, Whitelaw E. The vagaries of variegating transgenes. BioEssays. 1996;18:919–923. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

