Is adipose tissue a place for Mycobacterium tuberculosis persistence?
- PMID: 17183672
- PMCID: PMC1762355
- DOI: 10.1371/journal.pone.0000043
Is adipose tissue a place for Mycobacterium tuberculosis persistence?
Abstract
Background: Mycobacterium tuberculosis, the etiological agent of tuberculosis (TB), has the ability to persist in its human host for exceptionally long periods of time. However, little is known about the location of the bacilli in latently infected individuals. Long-term mycobacterial persistence in the lungs has been reported, but this may not sufficiently account for strictly extra-pulmonary TB, which represents 10-15% of the reactivation cases.
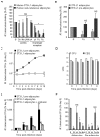
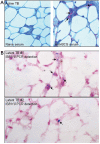
Methodology/principal findings: We applied in situ and conventional PCR to sections of adipose tissue samples of various anatomical origins from 19 individuals from Mexico and 20 from France who had died from causes other than TB. M. tuberculosis DNA could be detected by either or both techniques in fat tissue surrounding the kidneys, the stomach, the lymph nodes, the heart and the skin in 9/57 Mexican samples (6/19 individuals), and in 8/26 French samples (6/20 individuals). In addition, mycobacteria could be immuno-detected in perinodal adipose tissue of 1 out of 3 biopsy samples from individuals with active TB. In vitro, using a combination of adipose cell models, including the widely used murine adipose cell line 3T3-L1, as well as primary human adipocytes, we show that after binding to scavenger receptors, M. tuberculosis can enter within adipocytes, where it accumulates intracytoplasmic lipid inclusions and survives in a non-replicating state that is insensitive to the major anti-mycobacterial drug isoniazid.
Conclusions/significance: Given the abundance and the wide distribution of the adipose tissue throughout the body, our results suggest that this tissue, among others, might constitute a vast reservoir where the tubercle bacillus could persist for long periods of time, and avoid both killing by antimicrobials and recognition by the host immune system. In addition, M. tuberculosis-infected adipocytes might provide a new model to investigate dormancy and to evaluate new drugs for the treatment of persistent infection.
Conflict of interest statement
Figures

References
-
- Kaufmann SH. How can immunology contribute to the control of tuberculosis? Nat Rev Immunol. 2001;1:20–30. - PubMed
-
- Frieden TR, Sterling TR, Munsiff SS, Watt CJ, Dye C. Tuberculosis. Lancet. 2003;362:887–899. - PubMed
-
- Fine PE, Small PM. Exogenous reinfection in tuberculosis. N Engl J Med. 1999;341:1226–1227. - PubMed
-
- van Rie A, Warren R, Richardson M, Victor TC, Gie RP, et al. Exogenous reinfection as a cause of recurrent tuberculosis after curative treatment. N Engl J Med. 1999;341:1174–1179. - PubMed
-
- Parrish NM, Dick JD, Bishai WR. Mechanisms of latency in Mycobacterium tuberculosis. Trends Microbiol. 1998;6:107–112. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

