Catalases are NAD(P)H-dependent tellurite reductases
- PMID: 17183702
- PMCID: PMC1762332
- DOI: 10.1371/journal.pone.0000070
Catalases are NAD(P)H-dependent tellurite reductases
Abstract
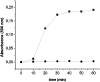
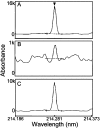
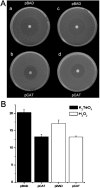
Reactive oxygen species damage intracellular targets and are implicated in cancer, genetic disease, mutagenesis, and aging. Catalases are among the key enzymatic defenses against one of the most physiologically abundant reactive oxygen species, hydrogen peroxide. The well-studied, heme-dependent catalases accelerate the rate of the dismutation of peroxide to molecular oxygen and water with near kinetic perfection. Many catalases also bind the cofactors NADPH and NADH tenaciously, but, surprisingly, NAD(P)H is not required for their dismutase activity. Although NAD(P)H protects bovine catalase against oxidative damage by its peroxide substrate, the catalytic role of the nicotinamide cofactor in the function of this enzyme has remained a biochemical mystery to date. Anions formed by heavy metal oxides are among the most highly reactive, natural oxidizing agents. Here, we show that a natural isolate of Staphylococcus epidermidis resistant to tellurite detoxifies this anion thanks to a novel activity of its catalase, and that a subset of both bacterial and mammalian catalases carry out the NAD(P)H-dependent reduction of soluble tellurite ion (TeO(3)(2-)) to the less toxic, insoluble metal, tellurium (Te(o)), in vitro. An Escherichia coli mutant defective in the KatG catalase/peroxidase is sensitive to tellurite, and expression of the S. epidermidis catalase gene in a heterologous E. coli host confers increased resistance to tellurite as well as to hydrogen peroxide in vivo, arguing that S. epidermidis catalase provides a physiological line of defense against both of these strong oxidizing agents. Kinetic studies reveal that bovine catalase reduces tellurite with a low Michaelis-Menten constant, a result suggesting that tellurite is among the natural substrates of this enzyme. The reduction of tellurite by bovine catalase occurs at the expense of producing the highly reactive superoxide radical.
Conflict of interest statement
Figures
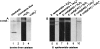

References
-
- McCord JM, Fridovich I. The utility of superoxide dismutase in studying free radical reactions. I. Radicals generated by the interaction of sulfite, dimethyl sulfoxide, and oxygen. J Biol Chem. 1969;244:6056–6063. - PubMed
-
- Nicholls P, Schonbaum GR. Catalases. In: Boyer P, Lardy H, Myrback K, editors. The Enzymes. New York and London: Academic press; 1963. pp. 147–225.
-
- Leow O. Physiological studies of Connecticut leaf tobacco. Rept US Dep of Agr Div of Veg Res. 1900;65:5–57.
-
- Sumner J, Dounce AL. Crystalline catalase. J Biol Chem. 1937;121:417–424.
-
- Lardinois OM. Reactions of bovine liver catalase with superoxide radicals and hydrogen peroxide. Free Radic Res. 1995;22:251–274. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

