GATA transcription factor required for immunity to bacterial and fungal pathogens
- PMID: 17183709
- PMCID: PMC1762309
- DOI: 10.1371/journal.pone.0000077
GATA transcription factor required for immunity to bacterial and fungal pathogens
Abstract
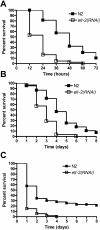
In the past decade, Caenorhabditis elegans has been used to dissect several genetic pathways involved in immunity; however, little is known about transcription factors that regulate the expression of immune effectors. C. elegans does not appear to have a functional homolog of the key immune transcription factor NF-kappaB. Here we show that that the intestinal GATA transcription factor ELT-2 is required for both immunity to Salmonella enterica and expression of a C-type lectin gene, clec-67, which is expressed in the intestinal cells and is a good marker of S. enterica infection. We also found that ELT-2 is required for immunity to Pseudomonas aeruginosa, Enterococcus faecalis, and Cryptococcus neoformans. Lack of immune inhibition by DAF-2, which negatively regulates the FOXO transcription factor DAF-16, rescues the hypersusceptibility to pathogens phenotype of elt-2(RNAi) animals. Our results indicate that ELT-2 is part of a multi-pathogen defense pathway that regulates innate immunity independently of the DAF-2/DAF-16 signaling pathway.
Conflict of interest statement
Figures

Similar articles
-
Pseudomonas aeruginosa suppresses host immunity by activating the DAF-2 insulin-like signaling pathway in Caenorhabditis elegans.PLoS Pathog. 2008 Oct;4(10):e1000175. doi: 10.1371/journal.ppat.1000175. Epub 2008 Oct 17. PLoS Pathog. 2008. PMID: 18927620 Free PMC article.
-
A conserved role for a GATA transcription factor in regulating epithelial innate immune responses.Proc Natl Acad Sci U S A. 2006 Sep 19;103(38):14086-91. doi: 10.1073/pnas.0603424103. Epub 2006 Sep 12. Proc Natl Acad Sci U S A. 2006. PMID: 16968778 Free PMC article.
-
Mode of bacterial pathogenesis determines phenotype in elt-2 and elt-7 RNAi Caenorhabditis elegans.Dev Comp Immunol. 2011 May;35(5):521-4. doi: 10.1016/j.dci.2010.12.008. Epub 2010 Dec 17. Dev Comp Immunol. 2011. PMID: 21168435
-
Strength in numbers: "Omics" studies of C. elegans innate immunity.Virulence. 2012 Oct 1;3(6):477-84. doi: 10.4161/viru.21906. Epub 2012 Oct 1. Virulence. 2012. PMID: 23076279 Free PMC article. Review.
-
Transcriptional responses to pathogens in Caenorhabditis elegans.Curr Opin Microbiol. 2008 Jun;11(3):251-6. doi: 10.1016/j.mib.2008.05.014. Epub 2008 Jun 21. Curr Opin Microbiol. 2008. PMID: 18567532 Free PMC article. Review.
Cited by
-
Take a Walk to the Wild Side of Caenorhabditis elegans-Pathogen Interactions.Microbiol Mol Biol Rev. 2021 Mar 17;85(2):e00146-20. doi: 10.1128/MMBR.00146-20. Print 2021 May 19. Microbiol Mol Biol Rev. 2021. PMID: 33731489 Free PMC article. Review.
-
C. elegans germline-deficient mutants respond to pathogen infection using shared and distinct mechanisms.PLoS One. 2010 Jul 26;5(7):e11777. doi: 10.1371/journal.pone.0011777. PLoS One. 2010. PMID: 20668681 Free PMC article.
-
Expression profiling and cross-species RNA interference (RNAi) of desiccation-induced transcripts in the anhydrobiotic nematode Aphelenchus avenae.BMC Mol Biol. 2010 Jan 19;11:6. doi: 10.1186/1471-2199-11-6. BMC Mol Biol. 2010. PMID: 20085654 Free PMC article.
-
TGF-β pathways in aging and immunity: lessons from Caenorhabditis elegans.Front Genet. 2023 Sep 5;14:1220068. doi: 10.3389/fgene.2023.1220068. eCollection 2023. Front Genet. 2023. PMID: 37732316 Free PMC article. Review.
-
GATA transcription factors as tissue-specific master regulators for induced responses.Worm. 2015 Nov 30;4(4):e1118607. doi: 10.1080/21624054.2015.1118607. eCollection 2015 Oct-Dec. Worm. 2015. PMID: 27123374 Free PMC article. Review.
References
-
- Hoffmann JA. The immune response of Drosophila. Nature. 2003;426:33–38. - PubMed
-
- Kim T, Kim YJ. Overview of innate immunity in Drosophila. J Biochem Mol Biol. 2005;38:121–127. - PubMed
-
- Kim DH, Ausubel FM. Evolutionary perspectives on innate immunity from the study of Caenorhabditis elegans. Curr Opin Immunol. 2005;17:4–10. - PubMed
-
- Sifri CD, Begun J, Ausubel FM. The worm has turned - microbial virulence modeled in Caenorhabditis elegans. Trends Microbiol. 2005;13:119–127. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

