A systems biology strategy reveals biological pathways and plasma biomarker candidates for potentially toxic statin-induced changes in muscle
- PMID: 17183729
- PMCID: PMC1762369
- DOI: 10.1371/journal.pone.0000097
A systems biology strategy reveals biological pathways and plasma biomarker candidates for potentially toxic statin-induced changes in muscle
Abstract
Background: Aggressive lipid lowering with high doses of statins increases the risk of statin-induced myopathy. However, the cellular mechanisms leading to muscle damage are not known and sensitive biomarkers are needed to identify patients at risk of developing statin-induced serious side effects.
Methodology: We performed bioinformatics analysis of whole genome expression profiling of muscle specimens and UPLC/MS based lipidomics analyses of plasma samples obtained in an earlier randomized trial from patients either on high dose simvastatin (80 mg), atorvastatin (40 mg), or placebo.
Principal findings: High dose simvastatin treatment resulted in 111 differentially expressed genes (1.5-fold change and p-value<0.05), while expression of only one and five genes was altered in the placebo and atorvastatin groups, respectively. The Gene Set Enrichment Analysis identified several affected pathways (23 gene lists with False Discovery Rate q-value<0.1) in muscle following high dose simvastatin, including eicosanoid synthesis and Phospholipase C pathways. Using lipidomic analysis we identified previously uncharacterized drug-specific changes in the plasma lipid profile despite similar statin-induced changes in plasma LDL-cholesterol. We also found that the plasma lipidomic changes following simvastatin treatment correlate with the muscle expression of the arachidonate 5-lipoxygenase-activating protein.
Conclusions: High dose simvastatin affects multiple metabolic and signaling pathways in skeletal muscle, including the pro-inflammatory pathways. Thus, our results demonstrate that clinically used high statin dosages may lead to unexpected metabolic effects in non-hepatic tissues. The lipidomic profiles may serve as highly sensitive biomarkers of statin-induced metabolic alterations in muscle and may thus allow us to identify patients who should be treated with a lower dose to prevent a possible toxicity.
Conflict of interest statement
Figures
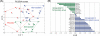


References
-
- Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl J Med. 1995;333:1301–1307. - PubMed
-
- Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, et al. The effect of Pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med. 1996;335:1001–1009. - PubMed
-
- Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344:1383–1389. - PubMed
-
- Bruckert E, Hayem G, Dejager S, Yau C, Begaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients - The PRIMO Study. Cardiovasc Drugs and Ther. 2005;19:403–414. - PubMed
-
- Schachter M. Chemical, pharmacokinetic and pharmacodynamic properties of statins: an update. Fundam Clin Pharmacol. 2005;19:117–125. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

