Divalent metal ion coordination by residue T118 of anthrax toxin receptor 2 is not essential for protective antigen binding
- PMID: 17183731
- PMCID: PMC1762376
- DOI: 10.1371/journal.pone.0000099
Divalent metal ion coordination by residue T118 of anthrax toxin receptor 2 is not essential for protective antigen binding
Abstract
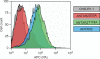
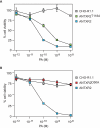
The protective antigen (PA) subunit of anthrax toxin interacts with the integrin-like I domains of either of two cellular receptors, ANTXR1 or ANTXR2. These I domains contain a metal ion-dependent adhesion site (MIDAS) made up of five non-consecutive amino acid residues that coordinate a divalent metal ion that is important for PA-binding. The MIDAS residues of integrin I domains shift depending upon whether the domain exists in a closed (ligand-unbound) or open (ligand-bound) conformation. Of relevance to this study, the MIDAS threonine residue coordinates the metal ion only in the open I domain conformation. Previously it was shown that the MIDAS threonine is essential for PA interaction with ANTXR1, a result consistent with the requirement that the I domain of that receptor adopts an open conformation for PA-binding. Here we have tested the requirement for the MIDAS threonine of ANTXR2 for PA-binding. We show that the toxin can bind to a mutant receptor lacking the MIDAS threonine and that it can use that mutant receptor to intoxicate cultured cells. These findings suggest that an open-like configuration of the ANTXR2 MIDAS is not essential for the interaction with PA.
Conflict of interest statement
Figures


Similar articles
-
Anthrax toxin receptor 1/tumor endothelial marker 8: mutation of conserved inserted domain residues overrides cytosolic control of protective antigen binding.Biochemistry. 2010 Aug 31;49(34):7403-10. doi: 10.1021/bi100887w. Biochemistry. 2010. PMID: 20690680 Free PMC article.
-
The cytoplasmic domain of anthrax toxin receptor 1 affects binding of the protective antigen.Infect Immun. 2009 Jan;77(1):52-9. doi: 10.1128/IAI.01073-08. Epub 2008 Oct 20. Infect Immun. 2009. PMID: 18936178 Free PMC article.
-
Human capillary morphogenesis protein 2 functions as an anthrax toxin receptor.Proc Natl Acad Sci U S A. 2003 Apr 29;100(9):5170-4. doi: 10.1073/pnas.0431098100. Epub 2003 Apr 16. Proc Natl Acad Sci U S A. 2003. PMID: 12700348 Free PMC article.
-
Interactions between anthrax toxin receptors and protective antigen.Curr Opin Microbiol. 2005 Feb;8(1):106-12. doi: 10.1016/j.mib.2004.12.005. Curr Opin Microbiol. 2005. PMID: 15694864 Review.
-
Roles of Anthrax Toxin Receptor 2 in Anthrax Toxin Membrane Insertion and Pore Formation.Toxins (Basel). 2016 Jan 22;8(2):34. doi: 10.3390/toxins8020034. Toxins (Basel). 2016. PMID: 26805886 Free PMC article. Review.
Cited by
-
Differential dependence on N-glycosylation of anthrax toxin receptors CMG2 and TEM8.PLoS One. 2015 Mar 17;10(3):e0119864. doi: 10.1371/journal.pone.0119864. eCollection 2015. PLoS One. 2015. PMID: 25781883 Free PMC article.
-
Receptors of anthrax toxin and cell entry.Mol Aspects Med. 2009 Dec;30(6):406-12. doi: 10.1016/j.mam.2009.08.007. Epub 2009 Sep 2. Mol Aspects Med. 2009. PMID: 19732789 Free PMC article. Review.
-
Binding of the von Willebrand Factor A Domain of Capillary Morphogenesis Protein 2 to Anthrax Protective Antigen Vaccine Reduces Immunogenicity in Mice.mSphere. 2020 Jan 15;5(1):e00556-19. doi: 10.1128/mSphere.00556-19. mSphere. 2020. PMID: 31941807 Free PMC article.
-
Anthrax toxin receptor 1/tumor endothelial marker 8: mutation of conserved inserted domain residues overrides cytosolic control of protective antigen binding.Biochemistry. 2010 Aug 31;49(34):7403-10. doi: 10.1021/bi100887w. Biochemistry. 2010. PMID: 20690680 Free PMC article.
-
Hyaline fibromatosis syndrome inducing mutations in the ectodomain of anthrax toxin receptor 2 can be rescued by proteasome inhibitors.EMBO Mol Med. 2011 Apr;3(4):208-21. doi: 10.1002/emmm.201100124. Epub 2011 Feb 15. EMBO Mol Med. 2011. PMID: 21328543 Free PMC article.
References
-
- Bradley KA, Mogridge J, Jonah G, Rainey A, Batty S, et al. Binding of anthrax toxin to its receptor is similar to alpha integrin-ligand interactions. J Biol Chem. 2003;278:49342–49347. - PubMed
-
- Collier RJ, Young JA. Anthrax toxin. Annu Rev Cell Dev Biol. 2003;19:45–70. - PubMed
-
- Bradley KA, Mogridge J, Mourez M, Collier RJ, Young JA. Identification of the cellular receptor for anthrax toxin. Nature. 2001;414:225–229. - PubMed
-
- Shimaoka M, Takagi J, Springer TA. Conformational regulation of integrin structure and function. Annu Rev Biophys Biomol Struct. 2002;31:485–516. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

