Choroid plexus epithelial monolayers--a cell culture model from porcine brain
- PMID: 17184532
- PMCID: PMC1774582
- DOI: 10.1186/1743-8454-3-13
Choroid plexus epithelial monolayers--a cell culture model from porcine brain
Abstract
Background: The goal of the present study was to develop an in vitro choroid plexus (CP) epithelial cell culture model for studying transport of protein-mediated drug secretion from blood to cerebrospinal fluid (CSF) and vice versa.
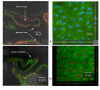
Methods: Cells were isolated by mechanical and enzymatic treatment of freshly isolated porcine plexus tissue. Epithelial cell monolayers were grown and CSF secretion and transepithelial resistance were determined. The expression of f-actin as well as the choroid plexus marker protein transthyretin (TTR), were assessed. The expression of the export proteins p-glycoprotein (Pgp, Abcb1) and multidrug resistance protein 1 (Mrp1, Abcc1) was studied by RT-PCR, Western-blot and immunofluorescence techniques and their functional activity was assessed by transport and uptake experiments.
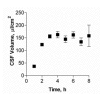
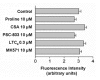
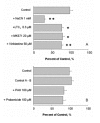
Results: Choroid plexus epithelial cells were isolated in high purity and grown to form confluent monolayers. Filter-grown monolayers displayed transendothelial resistance (TEER) values in the range of 100 to 150 ohms cm2. Morphologically, the cells showed the typical net work of f-actin and expressed TTR at a high rate. The cultured cells were able to secrete CSF at a rate of 48.2 +/- 4.6 microl/cm2/h over 2-3 hours. The ABC-export protein Mrp1 was expressed in the basolateral (blood-facing) membranes of cell monolayers and intact tissue. P-glycoprotein showed only low expression within the apical (CSF directed) membrane but was located more in sub-apical cell compartments. This finding was paralleled by the lack of directed excretion of p-glycoprotein substrates, verapamil and rhodamine 123.
Conclusion: It was demonstrated that CP epithelium can be isolated and cultured, with cells growing into intact monolayers, fully differentiating and with properties resembling the tissue in vivo. Thus, the established primary porcine CP model, allowing investigation of complex transport processes, can be used as a reliable tool for analysis of xenobiotic transport across the blood-cerebrospinal fluid barrier (BCSFB).
Figures






References
-
- Spector R, Johanson CE. The mammalian choroid plexus. Sci Am. 1989;261:68–74. - PubMed
-
- Cserr HF. Physiology of the choroid plexus. Physiol Rev. 1971;51:273–311. - PubMed
-
- el-Bacha RS, Minn A. Drug metabolizing enzymes in cerebrovascular endothelial cells afford a metabolic protection to the brain. Cell Mol Biol. 1999;45:15–23. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

