Tenofovir treatment augments anti-viral immunity against drug-resistant SIV challenge in chronically infected rhesus macaques
- PMID: 17184540
- PMCID: PMC1769512
- DOI: 10.1186/1742-4690-3-97
Tenofovir treatment augments anti-viral immunity against drug-resistant SIV challenge in chronically infected rhesus macaques
Abstract
Background: Emergence of drug-resistant strains of human immunodeficiency virus type 1 (HIV-1) is a major obstacle to successful antiretroviral therapy (ART) in HIV-infected patients. Whether antiviral immunity can augment ART by suppressing replication of drug-resistant HIV-1 in humans is not well understood, but can be explored in non-human primates infected with simian immunodeficiency virus (SIV). Rhesus macaques infected with live, attenuated SIV develop robust SIV-specific immune responses but remain viremic, often at low levels, for periods of months to years, thus providing a model in which to evaluate the contribution of antiviral immunity to drug efficacy. To investigate the extent to which SIV-specific immune responses augment suppression of drug-resistant SIV, rhesus macaques infected with live, attenuated SIVmac239Deltanef were treated with the reverse transcriptase (RT) inhibitor tenofovir, and then challenged with pathogenic SIVmac055, which has a five-fold reduced sensitivity to tenofovir.
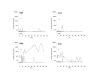
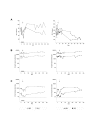
Results: Replication of SIVmac055 was detected in untreated macaques infected with SIVmac239Deltanef, and in tenofovir-treated, naïve control macaques. The majority of macaques infected with SIVmac055 experienced high levels of plasma viremia, rapid CD4+ T cell loss and clinical disease progression. By comparison, macaques infected with SIVmac239Deltanef and treated with tenofovir showed no evidence of replicating SIVmac055 in plasma using allele-specific real-time PCR assays with a limit of sensitivity of 50 SIV RNA copies/ml plasma. These animals remained clinically healthy with stable CD4+ T cell counts during three years of follow-up. Both the tenofovir-treated and untreated macaques infected with SIVmac239Deltanef had antibody responses to SIV gp130 and p27 antigens and SIV-specific CD8+ T cell responses prior to SIVmac055 challenge, but only those animals receiving concurrent treatment with tenofovir resisted infection with SIVmac055.
Conclusion: These results support the concept that anti-viral immunity acts synergistically with ART to augment drug efficacy by suppressing replication of viral variants with reduced drug sensitivity. Treatment strategies that seek to combine immunotherapeutic intervention as an adjunct to antiretroviral drugs may therefore confer added benefit by controlling replication of HIV-1, and reducing the likelihood of treatment failure due to the emergence of drug-resistant virus, thereby preserving treatment options.
Figures
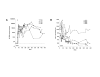


Similar articles
-
Prolonged tenofovir treatment of macaques infected with K65R reverse transcriptase mutants of SIV results in the development of antiviral immune responses that control virus replication after drug withdrawal.Retrovirology. 2012 Jul 17;9:57. doi: 10.1186/1742-4690-9-57. Retrovirology. 2012. PMID: 22805180 Free PMC article.
-
Sequential emergence and clinical implications of viral mutants with K70E and K65R mutation in reverse transcriptase during prolonged tenofovir monotherapy in rhesus macaques with chronic RT-SHIV infection.Retrovirology. 2007 Apr 6;4:25. doi: 10.1186/1742-4690-4-25. Retrovirology. 2007. PMID: 17417971 Free PMC article.
-
Prevention of SIV rectal transmission and priming of T cell responses in macaques after local pre-exposure application of tenofovir gel.PLoS Med. 2008 Aug 5;5(8):e157; discussion e157. doi: 10.1371/journal.pmed.0050157. PLoS Med. 2008. PMID: 18684007 Free PMC article.
-
Cannabinoid administration attenuates the progression of simian immunodeficiency virus.AIDS Res Hum Retroviruses. 2011 Jun;27(6):585-92. doi: 10.1089/aid.2010.0218. Epub 2010 Nov 23. AIDS Res Hum Retroviruses. 2011. PMID: 20874519 Free PMC article. Review.
-
The role of disease stage, plasma viral load and regulatory T cells (Tregs) on autoantibody production in SIV-infected non-human primates.J Autoimmun. 2007 Mar-May;28(2-3):152-9. doi: 10.1016/j.jaut.2007.02.014. Epub 2007 Mar 26. J Autoimmun. 2007. PMID: 17368846 Free PMC article. Review.
Cited by
-
Control of M184V HIV-1 mutants by CD8 T-cell responses.Med Microbiol Immunol. 2012 May;201(2):201-11. doi: 10.1007/s00430-011-0222-1. Epub 2011 Dec 27. Med Microbiol Immunol. 2012. PMID: 22200907
-
Chronic administration of tenofovir to rhesus macaques from infancy through adulthood and pregnancy: summary of pharmacokinetics and biological and virological effects.Antimicrob Agents Chemother. 2008 Sep;52(9):3144-60. doi: 10.1128/AAC.00350-08. Epub 2008 Jun 23. Antimicrob Agents Chemother. 2008. PMID: 18573931 Free PMC article.
-
Immuno-haematologic and virologic responses and predictors of virologic failure in HIV-1 infected adults on first-line antiretroviral therapy in Cameroon.Infect Dis Poverty. 2014 Jan 30;3(1):5. doi: 10.1186/2049-9957-3-5. Infect Dis Poverty. 2014. PMID: 24479873 Free PMC article.
-
Effects of the K65R and K65R/M184V reverse transcriptase mutations in subtype C HIV on enzyme function and drug resistance.Retrovirology. 2009 Feb 11;6:14. doi: 10.1186/1742-4690-6-14. Retrovirology. 2009. PMID: 19210791 Free PMC article.
-
Prolonged tenofovir treatment of macaques infected with K65R reverse transcriptase mutants of SIV results in the development of antiviral immune responses that control virus replication after drug withdrawal.Retrovirology. 2012 Jul 17;9:57. doi: 10.1186/1742-4690-9-57. Retrovirology. 2012. PMID: 22805180 Free PMC article.
References
-
- Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. - DOI - PubMed
-
- Phillips AN, Dunn D, Sabin C, Pozniak A, Matthias R, Geretti AM, Clarke J, Churchill D, Williams I, Hill T, et al. Long term probability of detection of HIV-1 drug resistance after starting antiretroviral therapy in routine clinical practice. AIDS. 2005;19:487–494. doi: 10.1097/01.aids.0000171414.99409.fb. - DOI - PubMed
-
- Barbour JD, Grant RM. The Role of Viral Fitness in HIV Pathogenesis. Curr HIV/AIDS Rep. 2005;2:29–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

