The shape of things to come: structural and synthetic studies of taxol and related compounds
- PMID: 17184797
- PMCID: PMC1979092
- DOI: 10.1016/j.phytochem.2006.11.009
The shape of things to come: structural and synthetic studies of taxol and related compounds
Abstract
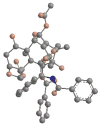
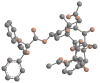
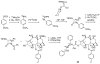
The history of the development of Taxol (paclitaxel) as an anticancer drug is reviewed, and some aspects of the phytochemistry of Taxus species and of the medicinal chemistry of taxol are discussed. The nature of the taxol-tubulin interaction is then described, with an emphasis on studies that led to the discovery and experimental proof of the T-taxol conformation as the tubulin-binding conformation of taxol. The implications of this conformation for future drug development are also briefly covered.
Figures

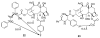
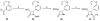
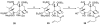
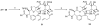
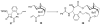
References
-
- Anonymous. Names for hi-jacking. Nature. 1995;373:370. - PubMed
-
- Baumann K. Neue mikrotubuli-stabilisatoren. Jenseits von paclitaxel und docetaxel. Pharm Unserer Zeit. 2005;34:110–114. - PubMed
-
- Chase M. The Wall Street Journal. New York: 1991. 9 April, Clashing priorities: A new cancer drug may extend lives - at cost of rare trees - that angers conservationists, who say taxol extraction endangers the prized yew - pressure on Bristol-Myers too; p. A1.
-
- Chaudhary AG, Rimoldi JM, Kingston DGI. Modified taxols. 10 Preparation of 7-deoxytaxol, a highly bioactive taxol derivative, and interconversions of taxol and 7-epitaxol. J Org Chem. 1993;58:3798–3799.
-
- Chaudhary AG, Kingston DGI. Synthesis of 10-deacetoxytaxol and 10-deoxytaxotere. Tetrahedron Lett. 1993;34:4921–4924.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

