Voltage-dependent calcium channels of dog basilar artery
- PMID: 17185332
- PMCID: PMC2075556
- DOI: 10.1113/jphysiol.2006.126128
Voltage-dependent calcium channels of dog basilar artery
Abstract
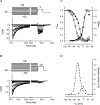
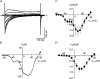
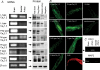
Electrophysiological and molecular characteristics of voltage-dependent calcium (Ca(2+)) channels were studied using whole-cell patch clamp, polymerase chain reaction and Western blotting in smooth muscle cells freshly isolated from dog basilar artery. Inward currents evoked by depolarizing steps from a holding potential of -50 or -90 mV in 10 mm barium consisted of low- (LVA) and high-voltage activated (HVA) components. LVA current comprised more than half of total current in 24 (12%) of 203 cells and less than 10% of total current in 52 (26%) cells. The remaining cells (127 cells, 62%) had LVA currents between one tenth and one half of total current. LVA current was rapidly inactivating, slowly deactivating, inhibited by high doses of nimodipine and mibefradil (> 0.3 microM), not affected by omega-agatoxin GVIA (gamma100 nM), omega-conotoxin IVA (1 microM) or SNX-482 (200 nM) and probably carried by T-type Ca(2+) channels based on the presence of messenger ribonucleic acid (mRNA) and protein for Ca(v3.1) and Ca(v3.3) alpha(1) subunits of these channels. LVA currents exhibited window current with a maximum of 13% of the LVA current at -37.4 mV. HVA current was slowly inactivating and rapidly deactivating. It was inhibited by nimodipine (IC(50) = 0.018 microM), mibefradil (IC(50) = 0.39 microM) and omega-conotoxin IV (1 microM). Smooth muscle cells also contained mRNA and protein for L- (Ca(v1.2) and Ca(v1.3)), N- (Ca(v2.2)) and T-type (Ca(v3.1) and Ca(v3.3)) alpha(1) Ca(2+) channel subunits. Confocal microscopy showed Ca(v1.2) and Ca(v1.3) (L-type), Ca(v2.2) (N-type) and Ca(v3.1) and Ca(v3.3) (T-type) protein in smooth muscle cells. Relaxation of intact arteries under isometric tension in vitro to nimodipine (1 microM) and mibefradil (1 microM) but not to omega-agatoxin GVIA (100 nM), omega-conotoxin IVA (1 microM) or SNX-482 (1 microM) confirmed the functional significance of L- and T-type voltage-dependent Ca(2+) channel subtypes but not N-type. These results show that dog basilar artery smooth muscle cells express functional voltage-dependent Ca(2+) channels of multiple types.
Figures





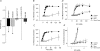
Similar articles
-
Alteration in voltage-dependent calcium channels in dog basilar artery after subarachnoid hemorrhage. Laboratory investigation.J Neurosurg. 2010 Oct;113(4):870-80. doi: 10.3171/2010.2.JNS091038. J Neurosurg. 2010. PMID: 20225918
-
Whole cell calcium currents in acutely isolated olfactory bulb output neurons of the rat.J Neurophysiol. 1996 Mar;75(3):1138-51. doi: 10.1152/jn.1996.75.3.1138. J Neurophysiol. 1996. PMID: 8867124
-
L, P-/Q- and T-type Ca2+ channels in smooth muscle cells from human umbilical artery.Cell Physiol Biochem. 2007;20(1-4):55-64. doi: 10.1159/000104153. Cell Physiol Biochem. 2007. PMID: 17595515
-
Calcium Channel Subtypes and Exocytosis in Chromaffin Cells at Early Life.Curr Mol Pharmacol. 2015;8(1):81-6. doi: 10.2174/1874467208666150507094537. Curr Mol Pharmacol. 2015. PMID: 25966701 Review.
-
Voltage-gated Ca(2+) channel mediated Ca(2+) influx in epileptogenesis.Adv Exp Med Biol. 2012;740:1219-47. doi: 10.1007/978-94-007-2888-2_55. Adv Exp Med Biol. 2012. PMID: 22453990 Review.
Cited by
-
Identification of L- and T-type Ca2+ channels in rat cerebral arteries: role in myogenic tone development.Am J Physiol Heart Circ Physiol. 2013 Jan 1;304(1):H58-71. doi: 10.1152/ajpheart.00476.2012. Epub 2012 Oct 26. Am J Physiol Heart Circ Physiol. 2013. PMID: 23103495 Free PMC article.
-
Calcium and potassium channels in experimental subarachnoid hemorrhage and transient global ischemia.Stroke Res Treat. 2012;2012:382146. doi: 10.1155/2012/382146. Epub 2012 Dec 9. Stroke Res Treat. 2012. PMID: 23251831 Free PMC article.
-
Importance of Altered Levels of SERCA, IP3R, and RyR in Vascular Smooth Muscle Cell.Biophys J. 2017 Jan 24;112(2):265-287. doi: 10.1016/j.bpj.2016.11.3206. Biophys J. 2017. PMID: 28122214 Free PMC article.
-
Intravascular pressure augments cerebral arterial constriction by inducing voltage-insensitive Ca2+ waves.J Physiol. 2010 Oct 15;588(Pt 20):3983-4005. doi: 10.1113/jphysiol.2010.193300. Epub 2010 Aug 24. J Physiol. 2010. PMID: 20736418 Free PMC article.
-
Dihydropyridine-insensitive calcium currents contribute to function of small cerebral arteries.J Cereb Blood Flow Metab. 2010 Jun;30(6):1226-39. doi: 10.1038/jcbfm.2010.11. Epub 2010 Feb 3. J Cereb Blood Flow Metab. 2010. PMID: 20125181 Free PMC article.
References
-
- Alborch E, Salom JB, Torregrosa G. Calcium channels in cerebral arteries. Pharmacol Ther. 1995;68:1–34. - PubMed
-
- Alderton JM, Steinhardt RA. Calcium influx through calcium leak channels is responsible for the elevated levels of calcium-dependent proteolysis in dystrophic myotubes. J Biol Chem. 2000;275:9452–9460. - PubMed
-
- Avdonin PV, Buhler FR, Tkachuk VA. Ca2+-agonistic effect of a T-type Ca-channel blocker mibefradil (Ro40-5967) Membr Cell Biol. 2000;13:645–655. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

