Changes in EMG coherence between long and short thumb abductor muscles during human development
- PMID: 17185340
- PMCID: PMC2075402
- DOI: 10.1113/jphysiol.2006.123174
Changes in EMG coherence between long and short thumb abductor muscles during human development
Abstract
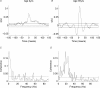
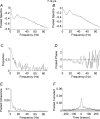
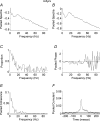
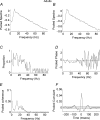
In adults, motoneurone pools of synergistic muscles that act around a common joint share a common presynaptic drive. Common drive can be revealed by both time domain and frequency domain analysis of EMG signals. Analysis in the frequency domain reveals significant coherence in the range 1-45 Hz, with maximal coherence in low (1-12 Hz) and high (16-32 Hz) ranges. The high-frequency range depends on cortical drive to motoneurones and is coherent with cortical oscillations at approximately 20 Hz frequencies. It is of interest to know whether oscillatory drive to human motoneurone pools changes with development. In the present study we examined age-related changes in coherence between rectified surface EMG signals recorded from the short and long thumb abductor muscles during steady isometric contraction obtained while subjects abducted the thumb against a manipulandum. We analysed EMG data from 36 subjects aged between 4 and 14 years, and 11 adult subjects aged between 22 and 59 years. Using the techniques of pooled coherence analysis and the chi(2) difference of coherence test we demonstrate that between the ages of 7 and 9 years, and 12 and 14 years, there are marked increases in the prevalence and magnitude of coherence at frequencies between 11 and 45 Hz. The data from subjects aged 12-14 years were similar to those obtained from adult controls. The most significant differences between younger children and the older age groups were detected at frequencies close to 20 Hz. We believe that these are the first reported results demonstrating significant late maturational changes in the approximately 20 Hz common oscillatory drive to human motoneurone pools.
Figures



Similar articles
-
On the development of human corticospinal oscillations: age-related changes in EEG-EMG coherence and cumulant.Eur J Neurosci. 2008 Jun;27(12):3369-79. doi: 10.1111/j.1460-9568.2008.06277.x. Eur J Neurosci. 2008. PMID: 18598272
-
Changes in cortically related intermuscular coherence accompanying improvements in locomotor skills in incomplete spinal cord injury.J Neurophysiol. 2006 Apr;95(4):2580-9. doi: 10.1152/jn.01289.2005. Epub 2006 Jan 11. J Neurophysiol. 2006. PMID: 16407422
-
Sensitivity of EMG-EMG coherence to detect the common oscillatory drive to hand muscles in young and older adults.J Neurophysiol. 2012 May;107(10):2866-75. doi: 10.1152/jn.01011.2011. Epub 2012 Feb 29. J Neurophysiol. 2012. PMID: 22378168
-
Muscle dependency of corticomuscular coherence in upper and lower limb muscles and training-related alterations in ballet dancers and weightlifters.J Appl Physiol (1985). 2010 Oct;109(4):1086-95. doi: 10.1152/japplphysiol.00869.2009. Epub 2010 Aug 5. J Appl Physiol (1985). 2010. PMID: 20689093
-
Rhythmical corticomotor communication.Neuroreport. 1999 Feb 5;10(2):R1-10. Neuroreport. 1999. PMID: 10203308 Review.
Cited by
-
Characteristics of multi-channel intermuscular directional coupling based on time-varying partial directional coherence analysis.Sci Rep. 2023 Oct 10;13(1):17088. doi: 10.1038/s41598-023-43976-0. Sci Rep. 2023. PMID: 37816900 Free PMC article.
-
Characterization of information-based learning benefits with submovement dynamics and muscular rhythmicity.PLoS One. 2013 Dec 18;8(12):e82920. doi: 10.1371/journal.pone.0082920. eCollection 2013. PLoS One. 2013. PMID: 24367568 Free PMC article. Clinical Trial.
-
Neural coupling between homologous muscles during bimanual tasks: effects of visual and somatosensory feedback.J Neurophysiol. 2017 Feb 1;117(2):655-664. doi: 10.1152/jn.00269.2016. Epub 2016 Nov 16. J Neurophysiol. 2017. PMID: 27852730 Free PMC article.
-
The human central nervous system transmits common synaptic inputs to distinct motor neuron pools during non-synergistic digit actions.J Physiol. 2019 Dec;597(24):5935-5948. doi: 10.1113/JP278623. Epub 2019 Oct 30. J Physiol. 2019. PMID: 31605381 Free PMC article.
-
Effect of Task Failure on Intermuscular Coherence Measures in Synergistic Muscles.Appl Bionics Biomech. 2018 Jun 3;2018:4759232. doi: 10.1155/2018/4759232. eCollection 2018. Appl Bionics Biomech. 2018. PMID: 29967654 Free PMC article.
References
-
- Amjad AM, Halliday D, Rosenberg JR, Conway BA. An extended difference of coherence test for comparing and combining several independent coherence estimates: theory and application to the study of motor units and physiological tremor. J Neurosci Methods. 1997;73:69–79. - PubMed
-
- Baker SN, Kilner JM, Pinches EM, Lemon RN. The role of synchrony and oscillations in the motor output. Exp Brain Res. 1999;128:109–117. - PubMed
-
- Brown P, Salenius S, Rothwell JC, Hari R. Cortical correlate of the Piper rhythm in humans. J Neurophysiol. 1998;80:2911–2917. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

