IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo
- PMID: 17185418
- PMCID: PMC1765450
- DOI: 10.1073/pnas.0606854104
IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo
Abstract
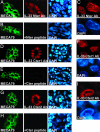
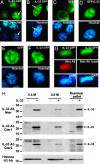
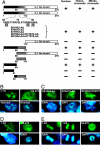
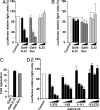
Recent studies indicate that IL-1alpha functions intracellularly in pathways independent of its cell surface receptors by translocating to the nucleus and regulating transcription. Similarly, the chromatin-associated protein HMGB1 acts as both a nuclear factor and a secreted proinflammatory cytokine. Here, we show that IL-33, an IL-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines, is an endothelium-derived, chromatin-associated nuclear factor with transcriptional repressor properties. We found that IL-33 is identical to NF-HEV, a nuclear factor preferentially expressed in high endothelial venules (HEV), that we previously characterized. Accordingly, in situ hybridization demonstrated that endothelial cells constitute a major source of IL-33 mRNA in chronically inflamed tissues from patients with rheumatoid arthritis and Crohn's disease. Immunostaining with three distinct antisera, directed against the N-terminal part and IL-1-like C-terminal domain, revealed that IL-33 is a heterochromatin-associated nuclear factor in HEV endothelial cells in vivo. Association of IL-33 with heterochromatin was also observed in human and mouse cells under living conditions. In addition, colocalization of IL-33 with mitotic chromatin was noted. Nuclear localization, heterochromatin-association, and targeting to mitotic chromosomes were all found to be mediated by an evolutionarily conserved homeodomain-like helix-turn-helix motif within the IL-33 N-terminal part. Finally, IL-33 was found to possess transcriptional repressor properties, associated with the homeodomain-like helix-turn-helix motif. Together, these data suggest that, similarly to IL1alpha and HMGB1, IL-33 is a dual function protein that may function as both a proinflammatory cytokine and an intracellular nuclear factor with transcriptional regulatory properties.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

