Regulation of Kv1 channel trafficking by the mamba snake neurotoxin dendrotoxin K
- PMID: 17185748
- PMCID: PMC2737685
- DOI: 10.1096/fj.06-7229com
Regulation of Kv1 channel trafficking by the mamba snake neurotoxin dendrotoxin K
Abstract
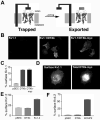
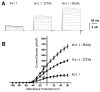
Modulation of voltage-gated potassium (Kv) channel surface expression can profoundly affect neuronal excitability. Some, but not all, mammalian Shaker or Kv1 alpha subunits contain a dominant endoplasmic reticulum (ER) retention signal in their pore region, preventing surface expression of Kv1.1 homotetrameric channels and of heteromeric Kv1 channels containing more than one Kv1.1 subunit. The critical amino acid residues within this ER pore-region retention signal are also critical for high-affinity binding of snake dendrotoxins (DTX). This suggests that ER retention may be mediated by an ER protein with a domain structurally similar to that of DTX. One facet of such a model is that expression of soluble DTX in the ER lumen should compete for binding to the retention protein and allow for surface expression of retained Kv1.1. Here, we show that luminal DTX expression dramatically increased both the level of cell surface Kv1.1 immunofluorescence staining and the proportion of Kv1.1 with processed N-linked oligosaccharides. Electrophysiological analyses showed that luminal DTX expression led to significant increases in Kv1.1 currents. Together, these data showed that luminal DTX expression increases surface expression of functional Kv1.1 homotetrameric channels and support a model whereby a DTX-like ER protein regulates abundance of cell surface Kv1 channels.
Figures


References
-
- Hille B. Ionic channels of excitable membranes. Sinauer; Sunderland, MA: 2001.
-
- Manganas LN, Akhtar S, Antonucci DE, Campomanes CR, Dolly JO, Trimmer JS. Episodic ataxia type-1 mutations in the Kv1.1 potassium channel display distinct folding and intracellular trafficking properties. J Biol Chem. 2001;276:49427–49434. - PubMed
-
- Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, Moreno H, Nadal MS, Ozaita A, Pountney D, Saganich M, Vega-Saenz de Miera E, Rudy B. Molecular diversity of K+ channels. In: Rudy B, Seeburg P, editors. Molecular and functional diversity of ion channels and receptors. Vol. 868. 1999. pp. 233–285. - PubMed
- Ann N Y Acad Sci
-
- Smart SL, Lopantsev V, Zhang CL, Robbins CA, Wang H, Chiu SY, Schwartzkroin PA, Messing A, Tempel BL. Deletion of the K(V)1.1 potassium channel causes epilepsy in mice. Neuron. 1998;20:809–819. - PubMed
-
- Rettig J, Heinemann SH, Wunder F, Lorra C, Parcej DN, Dolly JO, Pongs O. Inactivation properties of voltage-gated K+ channels altered by presence of beta-subunit. Nature. 1994;369:289–294. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

