Mutations of presenilin genes in dilated cardiomyopathy and heart failure
- PMID: 17186461
- PMCID: PMC1698711
- DOI: 10.1086/509900
Mutations of presenilin genes in dilated cardiomyopathy and heart failure
Abstract
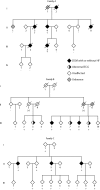
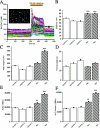
Two common disorders of the elderly are heart failure and Alzheimer disease (AD). Heart failure usually results from dilated cardiomyopathy (DCM). DCM of unknown cause in families has recently been shown to result from genetic disease, highlighting newly discovered disease mechanisms. AD is the most frequent neurodegenerative disease of older Americans. Familial AD is caused most commonly by presenilin 1 (PSEN1) or presenilin 2 (PSEN2) mutations, a discovery that has greatly advanced the field. The presenilins are also expressed in the heart and are critical to cardiac development. We hypothesized that mutations in presenilins may also be associated with DCM and that their discovery could provide new insight into the pathogenesis of DCM and heart failure. A total of 315 index patients with DCM were evaluated for sequence variation in PSEN1 and PSEN2. Families positive for mutations underwent additional clinical, genetic, and functional studies. A novel PSEN1 missense mutation (Asp333Gly) was identified in one family, and a single PSEN2 missense mutation (Ser130Leu) was found in two other families. Both mutations segregated with DCM and heart failure. The PSEN1 mutation was associated with complete penetrance and progressive disease that resulted in the necessity of cardiac transplantation or in death. The PSEN2 mutation showed partial penetrance, milder disease, and a more favorable prognosis. Calcium signaling was altered in cultured skin fibroblasts from PSEN1 and PSEN2 mutation carriers. These data indicate that PSEN1 and PSEN2 mutations are associated with DCM and heart failure and implicate novel mechanisms of myocardial disease.
Figures


References
Web Resources
-
- OHSU Familial Dilated Cardiomyopathy Research Project, http://www.fdc.to/
-
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for AD, PSEN1, PSEN2, DCM, LMNA, MYH7, TNNT2, SCN5A, CSRP3, and PLN) - PubMed
References
-
- Hunt SA (2005) ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). J Am Coll Cardiol 46:e1–e8210.1016/j.jacc.2005.08.022 - DOI - PubMed
-
- Rogaev EI, Sherrington R, Rogaeva EA, Levesque G, Ikeda M, Liang Y, Chi H, Lin C, Holman K, Tsuda T, Mar L, Sorbi S, Nacmias B, Piacentini S, Amaducci L, Chumakov I, Cohen D, Lannfelt L, Fraser PE, Rommens JM, St George-Hyslop PH (1995) Familial Alzheimer’s disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer’s disease type 3 gene. Nature 376:775–77810.1038/376775a0 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

