Evidence for BAG3 modulation of HIV-1 gene transcription
- PMID: 17187345
- PMCID: PMC2670777
- DOI: 10.1002/jcp.20865
Evidence for BAG3 modulation of HIV-1 gene transcription
Abstract
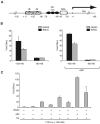
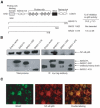
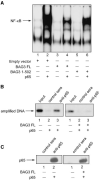
A family of co-chaperone proteins that share the Bcl-2-associated athanogene (BAG) domain are involved in a number of cellular processes, including proliferation and apoptosis. Among these proteins, BAG3 has received increased attention due to its high levels in several disease models and ability to associate with Hsp70 and a number of other molecular partners. BAG3 expression is stimulated during cell response to stressful conditions, such as exposure to high temperature, heavy metals, and certain drugs. Here, we demonstrate that BAG3 expression is elevated upon HIV-1 infection of human lymphocytes and fetal microglial cells. Furthermore, BAG3 protein was detectable in the cytoplasm of reactive astrocytes in HIV-1-associated encephalopathy biopsies, suggesting that induction of BAG3 is part of the host cell response to viral infection. To assess the impact of BAG3 upregulation on HIV-1 gene expression, we performed transcription assays and demonstrated that BAG3 can suppress transcription of the HIV-1 long terminal repeat (LTR) in microglial cells. This activity was mapped to the kappaB motif of the HIV-1 LTR. Results from in vitro and in vivo binding assays revealed that BAG3 suppresses interaction of the p65 subunit of NF-kappaB with the kappaB DNA motif of the LTR. Results from binding and transcriptional assay identified the C-terminus of BAG3 as a potential domain involved in the observed inhibitory effect of BAG3 on p65 activity. These observations reveal a previously unrecognized cell response, that is, an increase in BAG3, elicited by HIV-1 infection, and may provide a new avenue for the suppression of HIV-1 gene expression.
Copyright 2006 Wiley-Liss, Inc.
Figures

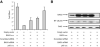
Similar articles
-
BAG3 protein regulates caspase-3 activation in HIV-1-infected human primary microglial cells.J Cell Physiol. 2009 Feb;218(2):264-7. doi: 10.1002/jcp.21604. J Cell Physiol. 2009. PMID: 18821563 Free PMC article.
-
HSP70 binding protein 1 (HspBP1) suppresses HIV-1 replication by inhibiting NF-κB mediated activation of viral gene expression.Nucleic Acids Res. 2016 Feb 29;44(4):1613-29. doi: 10.1093/nar/gkv1151. Epub 2015 Nov 3. Nucleic Acids Res. 2016. PMID: 26538602 Free PMC article.
-
HIV-1 Tat increases BAG3 via NF-κB signaling to induce autophagy during HIV-associated neurocognitive disorder.Cell Cycle. 2018;17(13):1614-1623. doi: 10.1080/15384101.2018.1480219. Epub 2018 Aug 21. Cell Cycle. 2018. PMID: 29962275 Free PMC article.
-
Regulation of HIV-1 gene transcription: from lymphocytes to microglial cells.J Leukoc Biol. 2003 Nov;74(5):736-49. doi: 10.1189/jlb.0403180. Epub 2003 Aug 11. J Leukoc Biol. 2003. PMID: 12960235 Review.
-
BAG3: a multifaceted protein that regulates major cell pathways.Cell Death Dis. 2011 Apr 7;2(4):e141. doi: 10.1038/cddis.2011.24. Cell Death Dis. 2011. PMID: 21472004 Free PMC article. Review.
Cited by
-
BAG3 protein regulates caspase-3 activation in HIV-1-infected human primary microglial cells.J Cell Physiol. 2009 Feb;218(2):264-7. doi: 10.1002/jcp.21604. J Cell Physiol. 2009. PMID: 18821563 Free PMC article.
-
HIV-1 Tat protein induces glial cell autophagy through enhancement of BAG3 protein levels.Cell Cycle. 2014;13(23):3640-4. doi: 10.4161/15384101.2014.952959. Cell Cycle. 2014. PMID: 25483098 Free PMC article.
-
Identification of phosphatases that dephosphorylate the co-chaperone BAG3.Life Sci Alliance. 2024 Nov 19;8(2):e202402734. doi: 10.26508/lsa.202402734. Print 2025 Feb. Life Sci Alliance. 2024. PMID: 39562141 Free PMC article.
-
BAG-1 interacts with the p50-p50 homodimeric NF-κB complex: implications for colorectal carcinogenesis.Oncogene. 2012 May 31;31(22):2761-72. doi: 10.1038/onc.2011.452. Epub 2011 Oct 3. Oncogene. 2012. PMID: 21963853 Free PMC article.
-
Expression of Bis in the mouse gastrointestinal system.Anat Cell Biol. 2012 Sep;45(3):160-9. doi: 10.5115/acb.2012.45.3.160. Epub 2012 Sep 30. Anat Cell Biol. 2012. PMID: 23094204 Free PMC article.
References
-
- Albright AV, Soldan SS, Gonzalez-Scarano F. Pathogenesis of human immunodeficiency virus-induced neurological disease. J NeuroVirol. 2003;9:222–227. - PubMed
-
- Amini S, Clavo A, Nadraga Y, Giordano A, Khalili K, Sawaya BE. Interplay between cdk9 and NF-κB factors determines the level of HIV-1gene transcription in astrocytic cells. Oncogene. 2002;21:5797–5803. - PubMed
-
- Antoku K, Maser RS, Scully WJ, Jr, Delach SM, Johnson DE. Isolation of Bcl01 binding proteins that exhibit homology with BAG-1 and suppressor of death domains proteins. Biochem Biophys Res Comm. 2001;286:1003–1010. - PubMed
-
- Bonelli P, Putrella A, Rosati A, Romano MF, Lerose R, Pagliuca MG, Amelio T, Festa M, Martire G, Venuta S, Turco MC, Leone A. BAG3 protein regulates stress-induced apoptosis in normal and neoplastic leukocytes. Leukemia. 2004;18:358–360. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

