The role of cholesterol and sphingolipids in chemokine receptor function and HIV-1 envelope glycoprotein-mediated fusion
- PMID: 17187670
- PMCID: PMC1769366
- DOI: 10.1186/1743-422X-3-104
The role of cholesterol and sphingolipids in chemokine receptor function and HIV-1 envelope glycoprotein-mediated fusion
Abstract
Background: HIV-1 entry into cells is a multifaceted process involving target cell CD4 and the chemokine receptors, CXCR4 or CCR5. The lipid composition of the host cell plays a significant role in the HIV fusion process as it orchestrates the appropriate disposition of CD4 and co-receptors required for HIV-1 envelope glycoprotein (Env)-mediated fusion. The cell membrane is primarily composed of sphingolipids and cholesterol. The effects of lipid modulation on CD4 disposition in the membrane and their role in HIV-1 entry have extensively been studied. To focus on the role of lipid composition on chemokine receptor function, we have by-passed the CD4 requirement for HIV-1 Env-mediated fusion by using a CD4-independent strain of HIV-1 Env.
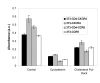
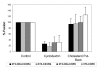
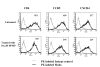
Results: Cell fusion mediated by a CD4-independent strain of HIV-1 Env was monitored by observing dye transfer between Env-expressing cells and NIH3T3 cells bearing CXCR4 or CCR5 in the presence or absence of CD4. Chemokine receptor signaling was assessed by monitoring changes in intracellular [Ca2+] mobilization induced by CCR5 or CXCR4 ligand. To modulate target membrane cholesterol or sphingolipids we used Methyl-beta-cyclodextrin (MbetaCD) or 1-phenyl-2-hexadecanoylamino-3-morpholino-1-propanol (PPMP), respectively. Treatment of the target cells with these agents did not change the levels of CD4 or CXCR4, but reduced levels of CCR5 on the cell surface. Chemokine receptor signalling was inhibited by cholesterol removal but not by treatment with PPMP. HIV-1 Env mediated fusion was inhibited by >50% by cholesterol removal. Overall, PPMP treatment appeared to slow down the rates of CD4-independent HIV-1 Env-mediated Fusion. However, in the case of CXCR4-dependent fusion, the differences between untreated and PPMP-treated cells did not appear to be significant.
Conclusion: Although modulation of cholesterol and sphingolipids has similar effects on CD4-dependent HIV-1 Env-mediated fusion, sphingolipid modulation had little effect on CD4-independent HIV-1 Env-mediated fusion. Chemokine receptor function remained intact following treatment of cells with PPMP. Therefore such treatment may be considered a more suitable agent to inhibit CD4 dependent HIV-1 infection.
Figures

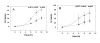

Similar articles
-
Functional expression of CD4, CXCR4, and CCR5 in glycosphingolipid-deficient mouse melanoma GM95 cells and susceptibility to HIV-1 envelope glycoprotein-triggered membrane fusion.Virology. 2004 Jan 5;318(1):55-65. doi: 10.1016/j.virol.2003.08.042. Virology. 2004. PMID: 14972535
-
Interactions of CCR5 and CXCR4 with CD4 and gp120 in human blood monocyte-derived dendritic cells.Exp Mol Pathol. 2000 Jun;68(3):133-8. doi: 10.1006/exmp.1999.2300. Exp Mol Pathol. 2000. PMID: 10816381
-
Use of GPR1, GPR15, and STRL33 as coreceptors by diverse human immunodeficiency virus type 1 and simian immunodeficiency virus envelope proteins.Virology. 1998 Sep 30;249(2):367-78. doi: 10.1006/viro.1998.9306. Virology. 1998. PMID: 9791028
-
Sphingolipids: modulators of HIV-1 infection and pathogenesis.Biosci Rep. 2005 Oct-Dec;25(5-6):329-43. doi: 10.1007/s10540-005-2894-5. Biosci Rep. 2005. PMID: 16307380 Review.
-
Mechanisms of apoptosis induction by the HIV-1 envelope.Cell Death Differ. 2005 Aug;12 Suppl 1:916-23. doi: 10.1038/sj.cdd.4401584. Cell Death Differ. 2005. PMID: 15719026 Review.
Cited by
-
The lipid membrane of HIV-1 stabilizes the viral envelope glycoproteins and modulates their sensitivity to antibody neutralization.J Biol Chem. 2020 Jan 10;295(2):348-362. doi: 10.1074/jbc.RA119.009481. Epub 2019 Nov 22. J Biol Chem. 2020. PMID: 31757809 Free PMC article.
-
Low cholesterol? Don't brag yet ... hypocholesterolemia blunts HAART effectiveness: a longitudinal study.J Int AIDS Soc. 2010 Jul 13;13:25. doi: 10.1186/1758-2652-13-25. J Int AIDS Soc. 2010. PMID: 20626901 Free PMC article. Clinical Trial.
-
Viral attachment induces rapid recruitment of an innate immune sensor (TRIM5α) to the plasma membrane.J Innate Immun. 2013;5(4):414-24. doi: 10.1159/000346963. Epub 2013 Mar 21. J Innate Immun. 2013. PMID: 23548691 Free PMC article.
-
Molecular Mechanism of HIV-1 Entry.Trends Microbiol. 2019 Oct;27(10):878-891. doi: 10.1016/j.tim.2019.06.002. Epub 2019 Jun 28. Trends Microbiol. 2019. PMID: 31262533 Free PMC article. Review.
-
Nuclear envelope remnants: fluid membranes enriched in sterols and polyphosphoinositides.PLoS One. 2009;4(1):e4255. doi: 10.1371/journal.pone.0004255. Epub 2009 Jan 23. PLoS One. 2009. PMID: 19165341 Free PMC article.
References
-
- Moulard M, Decroly E. Maturation of HIV envelope glycoprotein precursors by cellular endoproteases. Biochim Biophys Acta. 2000;1469:121–132. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

