Some considerations in the design and interpretation of antimalarial drug trials in uncomplicated falciparum malaria
- PMID: 17187673
- PMCID: PMC1769507
- DOI: 10.1186/1475-2875-5-127
Some considerations in the design and interpretation of antimalarial drug trials in uncomplicated falciparum malaria
Abstract
Background: Treatments for uncomplicated falciparum malaria should have high cure rates. The World Health Organization has recently set a target cure rate of 95% assessed at 28 days. The use of more effective drugs, with longer periods of patient follow-up, and parasite genotyping to distinguish recrudescence from reinfection raise issues related to the design and interpretation of antimalarial treatment trials in uncomplicated falciparum malaria which are discussed here.
Methods: The importance of adequate follow-up is presented and the advantages and disadvantages of non-inferiority trials are discussed. The different methods of interpreting trial results are described, and the difficulties created by loss to follow-up and missing or indeterminate genotyping results are reviewed.
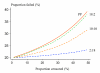
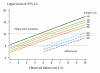
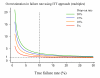
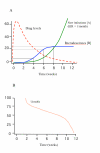
Conclusion: To characterize cure rates adequately assessment of antimalarial drug efficacy in uncomplicated malaria requires a minimum of 28 days and as much as 63 days follow-up after starting treatment. The longer the duration of follow-up in community-based assessments, the greater is the risk that this will be incomplete, and in endemic areas, the greater is the probability of reinfection. Recrudescence can be distinguished from reinfection using PCR genotyping but there are commonly missing or indeterminate results. There is no consensus on how these data should be analysed, and so a variety of approaches have been employed. It is argued that the correct approach to analysing antimalarial drug efficacy assessments is survival analysis, and patients with missing or indeterminate PCR results should either be censored from the analysis, or if there are sufficient data, results should be adjusted based on the identified ratio of new infections to recrudescences at the time of recurrent parasitaemia. Where the estimated cure rates with currently recommended treatments exceed 95%, individual comparisons with new regimens should generally be designed as non-inferiority trials with sample sizes sufficient to determine adequate precision of cure rate estimates (such that the lower 95% confidence interval bound exceeds 90%).
Figures
References
-
- World Health Organization Guidelines for the treatment of malaria. 2006. WHO/HTM/MAL/2006.1108.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

