At-level neuropathic pain is induced by lumbosacral ventral root avulsion injury and ameliorated by root reimplantation into the spinal cord
- PMID: 17187780
- PMCID: PMC2756497
- DOI: 10.1016/j.expneurol.2006.11.003
At-level neuropathic pain is induced by lumbosacral ventral root avulsion injury and ameliorated by root reimplantation into the spinal cord
Abstract
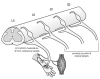
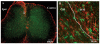
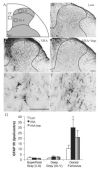
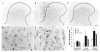
Neuropathic pain is common after traumatic injuries to the cauda equina/conus medullaris and brachial plexus. Clinically, this pain is difficult to treat and its mechanisms are not well understood. Lesions to the ventral roots are common in these injuries, but are rarely considered as potential contributors to pain. We examined whether a unilateral L6-S1 ventral root avulsion (VRA) injury in adult female rats might elicit pain within the dermatome projecting to the adjacent, uninjured L5 spinal segment. Additionally, a subset of subjects had the avulsed L6-S1 ventral roots reimplanted (VRA+Imp) into the lateral funiculus post-avulsion to determine whether this neural repair strategy elicits or ameliorates pain. Behavioral tests for mechanical allodynia and hyperalgesia were performed weekly over 7 weeks post-injury at the hindpaw plantar surface. Allodynia developed early and persisted post-VRA, whereas VRA+Imp rats exhibited allodynia only at 1 week post-operatively. Hyperalgesia was not observed at any time in any experimental group. Quantitative immunohistochemistry showed increased levels of inflammatory markers in laminae III-V and in the dorsal funiculus of the L5 spinal cord of VRA, but not VRA+Imp rats, specific to areas that receive projections from mechanoreceptive, but not nociceptive, primary afferents. These data suggest that sustained at-level neuropathic pain can develop following a pure motor lesion, whereas the pain may be ameliorated by acute root reimplantation. We believe that our findings are of translational research interest, as root implantation surgery is emerging as a potentially useful strategy for the repair of cauda equina/conus medullaris injuries.
Figures

References
-
- Aldskogius H. Microglia in neuroregeneration. Microsc Res Tech. 2001;54:40–46. - PubMed
-
- Anneser JM, Berthele A, Borasio GD, Castro-Lopes JM, Zieglgansberger W, Tolle TR. Axotomy of the sciatic nerve differentially affects expression of metabotropic glutamate receptor mRNA in adult rat motoneurons. Brain Res. 2000;868:215–221. - PubMed
-
- Back SK, Kim JS, Hong SK, Na HS. Ascending pathways for mechanical allodynia in a rat model of neuropathic pain. Neuroreport. 2003;14:1623–1626. - PubMed
-
- Berman JS, Birch R, Anand P. Pain following human brachial plexus injury with spinal cord root avulsion and the effect of surgery. Pain. 1998;75:199–207. - PubMed
-
- Cairns BE, Gambarota G, Svensson P, Arendt-Nielsen L, Berde CB. Glutamate-induced sensitization of rat masseter muscle fibers. Neuroscience. 2002;109:389–399. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

