In vitro and in vivo effects of bone marrow stem cells on cardiac structure and function
- PMID: 17187821
- PMCID: PMC1899533
- DOI: 10.1016/j.yjmcc.2006.10.009
In vitro and in vivo effects of bone marrow stem cells on cardiac structure and function
Abstract
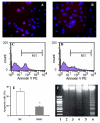
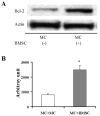
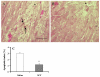
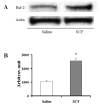
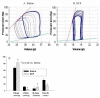
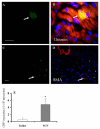
It is hypothesized that the protection of bone marrow stem cells (BMSCs) on ischemic myocardium might be related to the anti-apoptotic effect via paracrine mechanisms. In this study, a wide array of cytokines including vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), stromal cell-derived factor-1 (SDF-1) and insulin growth factor-1 (IGF-1) were detected in the BMSCs cultured medium by ELISA. Myocyte apoptosis was assayed by DNA fragmentation and annexin-V staining. Myocardial infarction model was produced by ligation of mouse left anterior descending coronary artery (LAD). Before LAD ligation, mice were myoablated by irradiation and transplanted with bone marrow cells from transgenic mice expressing green fluorescent protein (GFP). After LAD ligation, animals were administered stem cell factor (SCF, 200 mug/day/kg, i.p.) or saline for 6 days. Animals were sacrificed at 4 weeks after SCF treatment. Apoptotic cardiomyocytes were analyzed by TUNEL. Myocardial function was analyzed by echocardiography and pressure-volume system. Bcl-2 protein was analyzed by Western blotting. Our results showed that cultured BMSCs released VEGF, bFGF, SDF-1 and IGF-1. Hypoxia-induced cell apoptosis was diminished in cardiomyocytes co-cultured with BMSCs. Smaller LV dimension and increased LV ejection fraction were seen in SCF-treated animals. SCF significantly reduced cardiomyocytes apoptosis within peri-infarct area and increased up-regulation expression of Bcl-2 in ischemic area. Moreover, conditioned medium from cultured BMSCs also induced up-regulation of Bcl-2 protein in cardiomyocytes. It is concluded that paracrine mediators secreted by BMSCs might be involved in early repair of ischemic heart by preventing cardiomyocytes apoptosis and improving cardiac function.
Figures


Similar articles
-
Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling.Circ Res. 2006 Jun 9;98(11):1414-21. doi: 10.1161/01.RES.0000225952.61196.39. Epub 2006 May 11. Circ Res. 2006. PMID: 16690882
-
HIF-1alpha induced-VEGF overexpression in bone marrow stem cells protects cardiomyocytes against ischemia.J Mol Cell Cardiol. 2007 Jun;42(6):1036-44. doi: 10.1016/j.yjmcc.2007.04.001. Epub 2007 Apr 6. J Mol Cell Cardiol. 2007. PMID: 17498737 Free PMC article.
-
Myocardin-related transcription factor-A-overexpressing bone marrow stem cells protect cardiomyocytes and alleviate cardiac damage in a rat model of acute myocardial infarction.Int J Mol Med. 2015 Sep;36(3):753-9. doi: 10.3892/ijmm.2015.2261. Epub 2015 Jun 25. Int J Mol Med. 2015. PMID: 26135208
-
Stem cells in cardiac repair.Future Cardiol. 2011 Jan;7(1):99-117. doi: 10.2217/fca.10.109. Future Cardiol. 2011. PMID: 21174514 Review.
-
Cardiomyocyte differentiation of mesenchymal stem cells from bone marrow: new regulators and its implications.Stem Cell Res Ther. 2018 Feb 26;9(1):44. doi: 10.1186/s13287-018-0773-9. Stem Cell Res Ther. 2018. PMID: 29482607 Free PMC article. Review.
Cited by
-
The effects of mesenchymal stem cells on c-kit up-regulation and cell-cycle re-entry of neonatal cardiomyocytes are mediated by activation of insulin-like growth factor 1 receptor.Mol Cell Biochem. 2009 Dec;332(1-2):25-32. doi: 10.1007/s11010-009-0170-x. Epub 2009 Jun 9. Mol Cell Biochem. 2009. PMID: 19507001
-
VEGF is critical for stem cell-mediated cardioprotection and a crucial paracrine factor for defining the age threshold in adult and neonatal stem cell function.Am J Physiol Heart Circ Physiol. 2008 Dec;295(6):H2308-14. doi: 10.1152/ajpheart.00565.2008. Epub 2008 Oct 10. Am J Physiol Heart Circ Physiol. 2008. PMID: 18849336 Free PMC article.
-
The birth of 'regenerative pharmacology': a clinical perspective.Br J Pharmacol. 2013 May;169(2):239-46. doi: 10.1111/bph.12128. Br J Pharmacol. 2013. PMID: 23425309 Free PMC article. No abstract available.
-
Targeting human CD34+ hematopoietic stem cells with anti-CD45 x anti-myosin light-chain bispecific antibody preserves cardiac function in myocardial infarction.J Appl Physiol (1985). 2008 Jun;104(6):1793-800. doi: 10.1152/japplphysiol.01109.2007. Epub 2008 Feb 21. J Appl Physiol (1985). 2008. PMID: 18292296 Free PMC article.
-
Local renin-angiotensin system regulates hypoxia-induced vascular endothelial growth factor synthesis in mesenchymal stem cells.Int J Clin Exp Pathol. 2015 Mar 1;8(3):2505-14. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26045756 Free PMC article.
References
-
- Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–5. - PubMed
-
- Kudo M, Wang Y, Wani MA, Xu M, Ayub A, Ashraf M. Implantation of bone marrow stem cells reduces the infarction and fibrosis in ischemic mouse heart. J Mol Cell Cardiol. 2003;35:1113–9. - PubMed
-
- Strauer BE, Brehm M, Zeus T, Kostering M, Hernandez A, Sorg RV, et al. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation. 2002;106:1913–8. - PubMed
-
- Britten MB, Abolmaali ND, Assmus B, Lehmann R, Honold J, Schmitt J, et al. Infarct remodeling after intracoronary progenitor cell treatment in patients with acute myocardial infarction (TOPCARE-AMI): mechanistic insights from serial contrast-enhanced magnetic resonance imaging. Circulation. 2003;108:2212–8. - PubMed
-
- Shintani S, Murohara T, Ikeda H, Ueno T, Honma T, Katoh A, et al. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation. 2001;103:2776–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL 080686/HL/NHLBI NIH HHS/United States
- HL 70062/HL/NHLBI NIH HHS/United States
- R01 HL070062/HL/NHLBI NIH HHS/United States
- R01 HL083236/HL/NHLBI NIH HHS/United States
- HL 23597/HL/NHLBI NIH HHS/United States
- HL 074272/HL/NHLBI NIH HHS/United States
- HL 083236/HL/NHLBI NIH HHS/United States
- R01 HL081859/HL/NHLBI NIH HHS/United States
- R01 HL080686/HL/NHLBI NIH HHS/United States
- R01 HL091478/HL/NHLBI NIH HHS/United States
- HL 081859/HL/NHLBI NIH HHS/United States
- R37 HL074272/HL/NHLBI NIH HHS/United States
- R01 HL023597/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

