Exploring the mechanism of protein synthesis with modified substrates and novel intermediate mimics
- PMID: 17188006
- PMCID: PMC1810234
- DOI: 10.1016/j.bcmd.2006.11.002
Exploring the mechanism of protein synthesis with modified substrates and novel intermediate mimics
Abstract
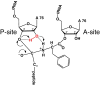
Translation, the synthesis of proteins from individual amino acids based on genetic information, is a cornerstone biological process. During ribosomal protein synthesis, new peptide bonds form through aminolysis of the peptidyl-tRNA ester bond by the alpha-amino group of the A-site amino acid. The rate of this reaction is accelerated at least 10(7)-fold in the ribosome, but the catalytic mechanism has remained controversial. We have used a combination of synthetic chemistry, biochemical, and structural biology approaches to characterize the mechanism of the peptidyl transfer reaction and the configuration of the reaction's tetrahedral intermediate. Substitution of the P-site tRNA A76 2' OH with 2' H or 2' F results in at least a 10(6)-fold reduction in the rate of peptide bond formation, but does not affect binding of the modified substrates. This indicates that the 2'-OH is essential to the reaction through participation in substrate assisted catalysis. A series of novel mimics of the tetrahedral intermediate were examined to distinguish between possible regio- and stereoisomeric forms of the intermediate. The determination of these parameters has important implications for the configuration of the substrates and intermediate within the ribosomal active site, and thus which functional groups are properly positioned to play various roles in promoting the reaction. Our results contribute to an emerging model of the peptidyl transfer reaction in which the ribosomal active site positions the substrates in an orientation specifically designed to promote the reaction, wherein the A76 2'-OH serves as a proton shuttle to enable critical proton transfers in the formation of the final peptide product.
Figures


References
-
- Green R, Noller HF. Ribosomes and translation. Annual Review of Biochemistry. 1997;66:679–716. - PubMed
-
- Fersht AR, Jencks WP. Reactions of Nucleophilic Reagents with Acylating Agents of Extreme Reactivity and Unreactivity - Correlation of Beta Values for Attacking and Leaving Group Variation. Journal of the American Chemical Society. 1970;92:5442–5452.
-
- Rodnina MV, Wintermeyer W. Peptide bond formation on the ribosome: structure and mechanism. Current Opinion in Structural Biology. 2003;13:334–40. - PubMed
-
- Fersht AR. Enzyme Structure and Mechanism. Freeman and Company; New York: 1985.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

