RNAi screen in mouse astrocytes identifies phosphatases that regulate NF-kappaB signaling
- PMID: 17188031
- PMCID: PMC2572259
- DOI: 10.1016/j.molcel.2006.10.015
RNAi screen in mouse astrocytes identifies phosphatases that regulate NF-kappaB signaling
Abstract
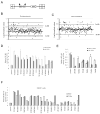
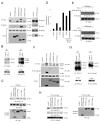
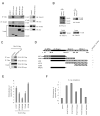
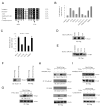
Regulation of NF-kappaB activation is controlled by a series of kinases; however, the roles of phosphatases in regulating this pathway are poorly understood. We report a systematic RNAi screen of phosphatases that modulate NF-kappaB activity. Nineteen of 250 phosphatase genes were identified as regulators of NF-kappaB signaling in astrocytes. RNAi selectively regulates endogenous chemokine and cytokine expression. Coimmunoprecipitation identified associations of distinct protein phosphatase 2A core or holoenzymes with the IKK, NF-kappaB, and TRAF2 complexes. Dephosphorylation of these complexes leads to modulation of NF-kappaB transcriptional activity. In contrast to IKK and NF-kappaB, TRAF2 phosphorylation has not been well elucidated. We show that the Thr117 residue in TRAF2 is phosphorylated following TNFalpha stimulation. This phosphorylation process is modulated by PP2A and is required for TRAF2 functional activity. These results provide direct evidence for TNF-induced TRAF2 phosphorylation and demonstrate that phosphorylation is regulated at multiple levels in the NF-kappaB pathway.
Figures

Similar articles
-
The protein phosphatase 2A regulatory subunit B56γ mediates suppression of T cell receptor (TCR)-induced nuclear factor-κB (NF-κB) activity.J Biol Chem. 2014 May 23;289(21):14996-5004. doi: 10.1074/jbc.M113.533547. Epub 2014 Apr 9. J Biol Chem. 2014. PMID: 24719332 Free PMC article.
-
TRAF2 phosphorylation promotes NF-κB-dependent gene expression and inhibits oxidative stress-induced cell death.Mol Biol Cell. 2011 Jan 1;22(1):128-40. doi: 10.1091/mbc.E10-06-0556. Epub 2010 Nov 30. Mol Biol Cell. 2011. PMID: 21119000 Free PMC article.
-
Tumor necrosis factor-alpha-induced IKK phosphorylation of NF-kappaB p65 on serine 536 is mediated through the TRAF2, TRAF5, and TAK1 signaling pathway.J Biol Chem. 2003 Sep 19;278(38):36916-23. doi: 10.1074/jbc.M301598200. Epub 2003 Jul 3. J Biol Chem. 2003. PMID: 12842894
-
Regulation and function of IKK and IKK-related kinases.Sci STKE. 2006 Oct 17;2006(357):re13. doi: 10.1126/stke.3572006re13. Sci STKE. 2006. PMID: 17047224 Review.
-
TRAF2 multitasking in TNF receptor-induced signaling to NF-κB, MAP kinases and cell death.Biochem Pharmacol. 2016 Sep 15;116:1-10. doi: 10.1016/j.bcp.2016.03.009. Epub 2016 Mar 16. Biochem Pharmacol. 2016. PMID: 26993379 Review.
Cited by
-
Interleukin-23 production in dendritic cells is negatively regulated by protein phosphatase 2A.Proc Natl Acad Sci U S A. 2010 May 4;107(18):8340-5. doi: 10.1073/pnas.0914703107. Epub 2010 Apr 19. Proc Natl Acad Sci U S A. 2010. PMID: 20404153 Free PMC article.
-
Basic principles and emerging concepts in the redox control of transcription factors.Antioxid Redox Signal. 2011 Oct 15;15(8):2335-81. doi: 10.1089/ars.2010.3534. Epub 2011 Apr 5. Antioxid Redox Signal. 2011. PMID: 21194351 Free PMC article. Review.
-
Endosomal Nox2 facilitates redox-dependent induction of NF-kappaB by TNF-alpha.Antioxid Redox Signal. 2009 Jun;11(6):1249-63. doi: 10.1089/ars.2008.2407. Antioxid Redox Signal. 2009. PMID: 19113817 Free PMC article.
-
The modulation of phosphatase expression impacts the proliferation efficiency of HSV-1 in infected astrocytes.PLoS One. 2013 Nov 15;8(11):e79648. doi: 10.1371/journal.pone.0079648. eCollection 2013. PLoS One. 2013. PMID: 24260274 Free PMC article.
-
Dephosphorylation of Carma1 by PP2A negatively regulates T-cell activation.EMBO J. 2011 Feb 2;30(3):594-605. doi: 10.1038/emboj.2010.331. Epub 2010 Dec 14. EMBO J. 2011. PMID: 21157432 Free PMC article.
References
-
- Bouwmeester T, Bauch A, Ruffner H, Angrand PO, Bergamini G, Croughton K, Cruciat C, Eberhard D, Gagneur J, Ghidelli S, et al. A physical and functional map of the human TNF-alpha/NF-kappa B signal transduction pathway. Nat Cell Biol. 2004;6:97–105. - PubMed
-
- Bureau F, Vanderplasschen A, Jaspar F, Minner F, Pastoret PP, Merville MP, Bours V, Lekeux P. Constitutive nuclear factor-kappaB activity preserves homeostasis of quiescent mature lymphocytes and granulocytes by controlling the expression of distinct Bcl-2 family proteins. Blood. 2002;99:3683–3691. - PubMed
-
- Bush TG, Puvanachandra N, Horner CH, Polito A, Ostenfeld T, Svendsen CN, Mucke L, Johnson MH, Sofroniew MV. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron. 1999;23:297–308. - PubMed
-
- Chaudhuri A, Orme S, Vo T, Wang W, Cherayil BJ. Phosphorylation of TRAF2 inhibits binding to the CD40 cytoplasmic domain. Biochem Biophys Res Commun. 1999;256:620–625. - PubMed
-
- DasGupta R, Kaykas A, Moon RT, Perrimon N. Functional genomic analysis of the Wnt-wingless signaling pathway. Science. 2005;308:826–833. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

