Pre-type 1 diabetes dysmetabolism: maximal sensitivity achieved with both oral and intravenous glucose tolerance testing
- PMID: 17188609
- PMCID: PMC1868416
- DOI: 10.1016/j.jpeds.2006.09.033
Pre-type 1 diabetes dysmetabolism: maximal sensitivity achieved with both oral and intravenous glucose tolerance testing
Abstract
Objective: To determine the relationship of intravenous (IVGTT) and oral (OGTT) glucose tolerance tests abnormalities to diabetes development in a high-risk pre-diabetic cohort and to identify an optimal testing strategy for detecting preclinical diabetes.
Study design: Diabetes Prevention Trial-Type 1 Diabetes (DPT-1) randomized subjects to oral (n = 372) and parenteral (n = 339) insulin prevention trials. Subjects were followed with IVGTTs and OGTTs. Factors associated with progression to diabetes were evaluated.
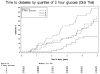
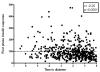
Results: Survival analysis revealed that higher quartiles of 2-hour glucose and lower quartiles of first phase insulin response (FPIR) at baseline were associated with decreased diabetes-free survival. Cox proportional hazards modeling showed that baseline body mass index (BMI), FPIR, and 2-hour glucose levels were significantly associated with an increased hazard for diabetes. On testing performed within 6 months of diabetes diagnosis, 3% (1/32) had normal FPIR and normal 2-hour glucose on OGTT. The sensitivities for impaired glucose tolerance (IGT) and low FPIR performed within 6 months of diabetes diagnosis were equivalent (76% vs 73%).
Conclusions: Most (97%) subjects had abnormal IVGTTs and/or OGTTs before the development of diabetes. The highest sensitivity is achieved using both tests.
Figures




References
-
- Lendrum R, Walker G, Gamble DR. Islet-cell antibodies in juvenile diabetes mellitus of recent onset. Lancet. 1975;7912:880–883. - PubMed
-
- Bottazzo GF, Dean BM, Gorsuch AN, Cudworth AG, Doniach D. Complement-fixing islet-cell antibodies in type I diabetes: possible monitors of active beta-cell damage. Lancet. 1980;8170:668–672. - PubMed
-
- Verge CF, Stenger D, Bonifacio E, Colman PG, Pilcher C, Bingley PJ, Eisenbarth GS. Combined use of autoantibodies (IA-2 autoantibody, GAD autoantibody, insulin autoantibody, cytoplasmic islet cell antibodies) in type 1 diabetes: Combinatorial Islet Autoantibody Workshop. Diabetes. 1998;47:1857–1866. - PubMed
-
- Yu L, Robles D, Rewers M, Kaur P, Keleman K, Eisenbarth GS. High-throughput insulin autoantibody assay: 96 well filtration plate format. Diabetes. 1999;48:A213.
-
- Gardner SG, Gale EA, Williams AJ, Gillespie KM, Lawrence KE, Bottazzo GF, Bingley PJ. Progression to diabetes in relatives with islet autoantibodies. Is it inevitable? Diabetes care. 1999;22:2049–2054. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

