Fast and selective ammonia transport by aquaporin-8
- PMID: 17189259
- PMCID: PMC3056221
- DOI: 10.1074/jbc.M609343200
Fast and selective ammonia transport by aquaporin-8
Abstract
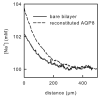
The transport of ammonia/ammonium is fundamental to nitrogen metabolism in all forms of life. So far, no clear picture has emerged as to whether a protein channel is capable of transporting exclusively neutral NH(3) while excluding H(+) and NH(4)(+). Our research is the first stoichiometric study to show the selective transport of NH(3) by a membrane channel. The purified water channel protein aquaporin-8 was reconstituted into planar bilayers, and the exclusion of NH(4)(+) or H(+) was established by ensuring a lack of current under voltage clamp conditions. The single channel water permeability coefficient of 1.2 x 10(-14) cm(3)/subunit/s was established by imposing an osmotic gradient across reconstituted planar bilayers, and resulting minute changes in ionic concentration close to the membrane surface were detected. It is more than 2-fold smaller than the single channel ammonia permeability (2.7 x 10(-14) cm(3)/subunit/s) that was derived by establishing a transmembrane ammonium concentration gradient and measuring the resulting concentration increases adjacent to the membrane. This permeability ratio suggests that electrically silent ammonia transport may be the main function of AQP8.
Figures




References
-
- King LS, Kozono D, Agre P. Nat. Rev. Mol. Cell Biol. 2004;5:687–698. - PubMed
-
- Yasui M, Hazama A, Kwon TH, Nielsen S, Guggino WB, Agre P. Nature. 1999;402:184–187. - PubMed
-
- Calamita G, Ferri D, Gena P, Liquori GE, Cavalier A, Thomas D, Svelto M. J. Biol. Chem. 2005;280:17149–17153. - PubMed
-
- Yang B, Zhao D, Verkman AS. J. Biol. Chem. 2006;281:16202–16206. - PubMed
-
- Yang B, Song Y, Zhao D, Verkman AS. Am. J. Physiol. 2005;288:C1161–C1170. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

