Subunit dependence of Na channel slow inactivation and open channel block in cerebellar neurons
- PMID: 17189307
- PMCID: PMC1861793
- DOI: 10.1529/biophysj.106.093500
Subunit dependence of Na channel slow inactivation and open channel block in cerebellar neurons
Abstract
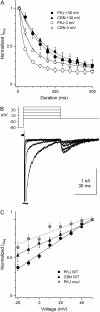
Purkinje and cerebellar nuclear neurons both have Na currents with resurgent kinetics. Previous observations, however, suggest that their Na channels differ in their susceptibility to entering long-lived inactivated states. To compare fast inactivation, slow inactivation, and open-channel block, we recorded voltage-clamped, tetrodotoxin-sensitive Na currents in Purkinje and nuclear neurons acutely isolated from mouse cerebellum. In nuclear neurons, recovery from all inactivated states was slower, and open-channel unblock was less voltage-dependent than in Purkinje cells. To test whether specific subunits contributed to this differential stability of inactivation, experiments were repeated in Na(V)1.6-null (med) mice. In med Purkinje cells, recovery times were prolonged and the voltage dependence of open-channel block was reduced relative to control cells, suggesting that availability of Na(V)1.6 is quickly restored at negative potentials. In med nuclear cells, however, currents were unchanged, suggesting that Na(V)1.6 contributes little to wild-type nuclear cells. Extracellular Na(+) prevented slow inactivation more effectively in Purkinje than in nuclear neurons, consistent with a resilience of Na(V)1.6 to slow inactivation. The tendency of nuclear Na channels to inactivate produced a low availability during trains of spike-like depolarization. Hyperpolarizations that approximated synaptic inhibition effectively recovered channels, suggesting that increases in Na channel availability promote rebound firing after inhibition.
Figures






References
-
- Thach, W. 1968. Discharge of Purkinje and cerebellar nuclear neurons during rapidly alternating arm movements in the monkey. J. Neurophysiol. 31:785–797. - PubMed
-
- McDevitt, C. J., T. J. Ebner, and J. R. Bloedel. 1987. Relationships between simultaneously recorded Purkinje cells and nuclear neurons. Brain Res. 425:1–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

