Silverleaf whitefly induces salicylic acid defenses and suppresses effectual jasmonic acid defenses
- PMID: 17189328
- PMCID: PMC1803729
- DOI: 10.1104/pp.106.090035
Silverleaf whitefly induces salicylic acid defenses and suppresses effectual jasmonic acid defenses
Abstract
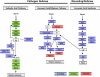
The basal defenses important in curtailing the development of the phloem-feeding silverleaf whitefly (Bemisia tabaci type B; SLWF) on Arabidopsis (Arabidopsis thaliana) were investigated. Sentinel defense gene RNAs were monitored in SLWF-infested and control plants. Salicylic acid (SA)-responsive gene transcripts accumulated locally (PR1, BGL2, PR5, SID2, EDS5, PAD4) and systemically (PR1, BGL2, PR5) during SLWF nymph feeding. In contrast, jasmonic acid (JA)- and ethylene-dependent RNAs (PDF1.2, VSP1, HEL, THI2.1, FAD3, ERS1, ERF1) were repressed or not modulated in SLWF-infested leaves. To test for a role of SA and JA pathways in basal defense, SLWF development on mutant and transgenic lines that constitutively activate or impair defense pathways was determined. By monitoring the percentage of SLWF nymphs in each instar, we show that mutants that activate SA defenses (cim10) or impair JA defenses (coi1) accelerated SLWF nymphal development. Reciprocally, mutants that activate JA defenses (cev1) or impair SA defenses (npr1, NahG) slowed SLWF nymphal development. Furthermore, when npr1 plants, which do not activate downstream SA defenses, were treated with methyl jasmonate, a dramatic delay in nymph development was observed. Collectively, these results showed that SLWF-repressed, JA-regulated defenses were associated with basal defense to the SLWF.
Figures





References
-
- Bouarab K, Melton R, Peart J, Baulcombe D, Osbourn A (2002) A saponin-detoxifying enzyme mediates suppression of plant defences. Nature 418 889–892 - PubMed
-
- Byrne DN, Bellows TS (1991) Whitefly biology. Annu Rev Entomol 36 431–457
-
- Chisholm ST, Coaker G, Day B, Staskawicz BJ (2006) Host-microbe interactions: shaping the evolution of the plant immune response. Cell 124 803–814 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

