Two Arabidopsis genes (IPMS1 and IPMS2) encode isopropylmalate synthase, the branchpoint step in the biosynthesis of leucine
- PMID: 17189332
- PMCID: PMC1803721
- DOI: 10.1104/pp.106.085555
Two Arabidopsis genes (IPMS1 and IPMS2) encode isopropylmalate synthase, the branchpoint step in the biosynthesis of leucine
Abstract
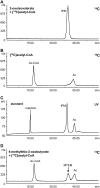
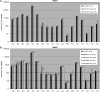
Heterologous expression of the Arabidopsis (Arabidopsis thaliana) IPMS1 (At1g18500) and IPMS2 (At1g74040) cDNAs in Escherichia coli yields isopropylmalate synthases (IPMSs; EC 2.3.3.13). These enzymes catalyze the first dedicated step in leucine (Leu) biosynthesis, an aldol-type condensation of acetyl-coenzyme A (CoA) and 2-oxoisovalerate yielding isopropylmalate. Most biochemical properties of IPMS1 and IPMS2 are similar: broad pH optimum around pH 8.5, Mg2+ as cofactor, feedback inhibition by Leu, Km for 2-oxoisovalerate of approximately 300 microM, and a Vmax of approximately 2 x 10(3) micromol min(-1) g(-1). However, IPMS1 and IPMS2 differ in their Km for acetyl-CoA (45 microM and 16 microM, respectively) and apparent quaternary structure (dimer and tetramer, respectively). A knockout insertion mutant for IPMS1 showed an increase in valine content but no changes in Leu content; two insertion mutants for IPMS2 did not show any changes in soluble amino acid content. Apparently, in planta each gene can adequately compensate for the absence of the other, consistent with available microarray and reverse transcription-polymerase chain reaction data that show that both genes are expressed in all organs at all developmental stages. Both encoded proteins accept 2-oxo acid substrates in vitro ranging in length from glyoxylate to 2-oxohexanoate, and catalyze at a low rate the condensation of acetyl-CoA and 4-methylthio-2-oxobutyrate, i.e. a reaction involved in glucosinolate chain elongation normally catalyzed by methylthioalkylmalate synthases. The evolutionary relationship between IPMS and methylthioalkylmalate synthase enzymes is discussed in view of their amino acid sequence identity (60%) and overlap in substrate specificity.
Figures





References
-
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301 653–657 - PubMed
-
- Baud S, Boutin J-P, Miquel M, Lepiniec L, Rochat C (2002) An integrated overview of seed development in Arabidopsis thaliana ecotype WS. Plant Physiol Biochem 40 151–160
-
- Bock CW, Katz AK, Markham GD, Glusker JP (1999) Manganese, a replacement for magnesium and zinc: functional comparison of the divalent ions. J Am Chem Soc 121 7360–7372
-
- Brown PD, Tokuhisa JG, Reichelt M, Gershenzon J (2003) Variation of glucosinolate accumulation among different organs and developmental stages of Arabidopsis thaliana. Phytochemistry 62 471–481 - PubMed
-
- Campos de Quiros H, Magrath R, McCallum D, Kroymann J, Schnabelrauch D, Mitchell-Olds T, Mithen R (2000) α-Keto acid elongation and glucosinolate biosynthesis in Arabidopsis thaliana. Theor Appl Genet 101 429–437
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

