On torque and tumbling in swimming Escherichia coli
- PMID: 17189361
- PMCID: PMC1855780
- DOI: 10.1128/JB.01501-06
On torque and tumbling in swimming Escherichia coli
Abstract
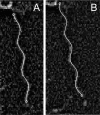
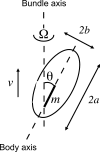
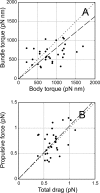
Bacteria swim by rotating long thin helical filaments, each driven at its base by a reversible rotary motor. When the motors of peritrichous cells turn counterclockwise (CCW), their filaments form bundles that drive the cells forward. We imaged fluorescently labeled cells of Escherichia coli with a high-speed charge-coupled-device camera (500 frames/s) and measured swimming speeds, rotation rates of cell bodies, and rotation rates of flagellar bundles. Using cells stuck to glass, we studied individual filaments, stopping their rotation by exposing the cells to high-intensity light. From these measurements we calculated approximate values for bundle torque and thrust and body torque and drag, and we estimated the filament stiffness. For both immobilized and swimming cells, the motor torque, as estimated using resistive force theory, was significantly lower than the motor torque reported previously. Also, a bundle of several flagella produced little more torque than a single flagellum produced. Motors driving individual filaments frequently changed directions of rotation. Usually, but not always, this led to a change in the handedness of the filament, which went through a sequence of polymorphic transformations, from normal to semicoiled to curly 1 and then, when the motor again spun CCW, back to normal. Motor reversals were necessary, although not always sufficient, to cause changes in filament chirality. Polymorphic transformations among helices having the same handedness occurred without changes in the sign of the applied torque.
Figures
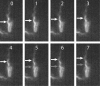
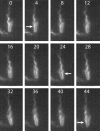
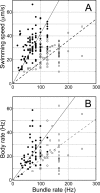

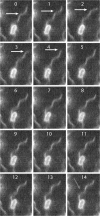

References
-
- Aizawa, S.-I. 2002. Flagella, p. 155-175. In M. Sussman (ed.), Molecular medical microbiology, vol. 1. Academic Press, San Diego, CA.
-
- Aizawa, S. I., and T. Kubori. 1998. Bacterial flagellation and cell division. Genes Cells 3:625-634. - PubMed
-
- Berg, H. C. 2004. E. coli in motion. Springer-Verlag, New York, NY.
-
- Berg, H. C. 1993. Random walks in biology, expanded edition. Princeton University Press, Princeton, NJ.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

