Growth phase regulation of Vibrio cholerae RTX toxin export
- PMID: 17189368
- PMCID: PMC1855747
- DOI: 10.1128/JB.01766-06
Growth phase regulation of Vibrio cholerae RTX toxin export
Abstract
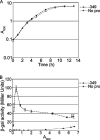
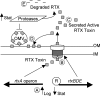
Vibrio cholerae, the causative agent of the severe diarrheal disease cholera, secretes several "accessory" toxins, including RTX toxin, which causes the cross-linking of the actin cytoskeleton. RTX toxin is exported to the extracellular milieu by an atypical type I secretion system (T1SS), and we previously noted that RTX-associated activity is detectable only in supernatant fluids from log phase cultures. Here, we investigate the mechanisms for regulating RTX toxin activity in supernatant fluids. We find that exported proteases are capable of destroying RTX activity and may therefore play a role in the growth phase regulation of toxin activity. We determined that the absence of RTX toxin in stationary-phase culture supernatant fluids is also due to a lack of toxin secretion and not attributable to solely proteolytic degradation. We ascertained that the T1SS apparatus is regulated at the transcriptional level by growth phase control that is independent of quorum sensing, unlike other virulence factors of V. cholerae. Additionally, in stationary-phase cultures, all RTX toxin activity is associated with bacterial membranes or outer membrane vesicles.
Figures







References
-
- Balsalobre, C., J. M. Silván, S. Berglund, Y. Mizunoe, B. E. Uhlin, and S. N. Wai. 2006. Release of the type I secreted α-haemolysin via outer membrane vesicles from Escherichia coli. Mol. Microbiol. 59:99-112. - PubMed
-
- Benítez, J. A., L. García, A. Silva, H. García, R. Fando, B. Cedré, A. Pérez, J. Campos, B. L. Rodríguez, J. L. Pérez, T. Valmaseda, O. Pérez, M. Ramírez, T. Ledón, M. D. Jidy, M. Lastre, L. Bravo, and G. Sierra. 1999. Preliminary assessment of the safety and immunogenicity of a new CTXφ-negative, hemagglutinin/protease-defective El Tor strain as a cholera vaccine candidate. Infect. Immun. 67:539-545. - PMC - PubMed
-
- Boardman, B. K., and K. J. Satchell. 2005. The Atypical TISS for secretion of Vibrio cholerae RTX toxin is regulated by growth phase, abstr. B-280, p. 81. Abstr. 105th Gen. Meet. Am. Soc. Microbiol., Atlanta, GA.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

