Weak rolling adhesion enhances bacterial surface colonization
- PMID: 17189376
- PMCID: PMC1855705
- DOI: 10.1128/JB.00899-06
Weak rolling adhesion enhances bacterial surface colonization
Abstract
Bacterial adhesion to and subsequent colonization of surfaces are the first steps toward forming biofilms, which are a major concern for implanted medical devices and in many diseases. It has generally been assumed that strong irreversible adhesion is a necessary step for biofilm formation. However, some bacteria, such as Escherichia coli when binding to mannosylated surfaces via the adhesive protein FimH, adhere weakly in a mode that allows them to roll across the surface. Since single-point mutations or even increased shear stress can switch this FimH-mediated adhesion to a strong stationary mode, the FimH system offers a unique opportunity to investigate the role of the strength of adhesion independently from the many other factors that may affect surface colonization. Here we compare levels of surface colonization by E. coli strains that differ in the strength of adhesion as a result of flow conditions or point mutations in FimH. We show that the weak rolling mode of surface adhesion can allow a more rapid spreading during growth on a surface in the presence of fluid flow. Indeed, an attempt to inhibit the adhesion of strongly adherent bacteria by blocking mannose receptors with a soluble inhibitor actually increased the rate of surface colonization by allowing the bacteria to roll. This work suggests that (i) a physiological advantage to the weak adhesion demonstrated by commensal variants of FimH bacteria may be to allow rapid surface colonization and (ii) antiadhesive therapies intended to prevent biofilm formation can have the unintended effect of enhancing the rate of surface colonization.
Figures
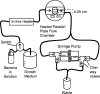
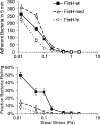


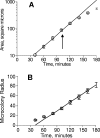
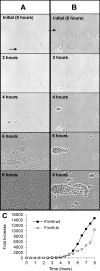

References
-
- Costerton, J. W., K. R. Rozee, and K. J. Cheng. 1983. Colonization of particulates, mucous, and intestinal tissue. Prog. Food Nutr. Sci. 7:91-105. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

