C-terminal loop of Streptomyces phospholipase D has multiple functional roles
- PMID: 17189478
- PMCID: PMC2203283
- DOI: 10.1110/ps.062537907
C-terminal loop of Streptomyces phospholipase D has multiple functional roles
Abstract
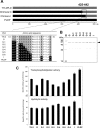
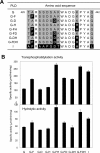
We have recently shown that two flexible loops of Streptomyces phospholipase D (PLD) affect the catalytic reaction of the enzyme by a comparative study of chimeric PLDs. Gly188 and Asp191 of PLD from Streptomyces septatus TH-2 (TH-2PLD) were identified as the key amino acid residues involved in the recognition of phospholipids. In the present study, we further investigated the relationship between a C-terminal loop of TH-2PLD and PLD activities to elucidate the reaction mechanism and the recognition of the substrate. By analyzing chimeras and mutants in terms of hydrolytic and transphosphatidylation activities, Ala426 and Lys438 of TH-2PLD were identified as the residues associated with the activities. We found that Gly188 and Asp191 recognized substrate forms, whereas residues Ala426 and Lys438 enhanced transphosphatidylation and hydrolysis activities regardless of the substrate form. By substituting Ala426 and Lys438 with Phe and His, respectively, the mutant showed not only higher activities but also higher thermostability and tolerance against organic solvents. Furthermore, the mutant also improved the selectivity of the transphosphatidylation activity. The residues Ala426 and Lys438 were located in the C-terminal flexible loop of Streptomyces PLD separate from the highly conserved catalytic HxKxxxxD motifs. We demonstrated that this C-terminal loop, which formed the entrance of the active well, has multiple functional roles in Streptomyces PLD.
Figures






Similar articles
-
Sensor of phospholipids in Streptomyces phospholipase D.FEBS J. 2007 May;274(10):2672-81. doi: 10.1111/j.1742-4658.2007.05802.x. Epub 2007 Apr 25. FEBS J. 2007. PMID: 17459102
-
Recognition of phospholipids in Streptomyces phospholipase D.J Biol Chem. 2005 Jul 15;280(28):26143-51. doi: 10.1074/jbc.M414319200. Epub 2005 May 17. J Biol Chem. 2005. PMID: 15899903
-
Study on thermostability of phospholipase D from Streptomyces sp.Biochim Biophys Acta. 2002 Jul 29;1598(1-2):156-64. doi: 10.1016/s0167-4838(02)00363-1. Biochim Biophys Acta. 2002. PMID: 12147356
-
Phospholipase D mechanism using Streptomyces PLD.Biochim Biophys Acta. 2009 Sep;1791(9):962-9. doi: 10.1016/j.bbalip.2009.01.020. Epub 2009 Feb 4. Biochim Biophys Acta. 2009. PMID: 19416643 Review.
-
Phospholipase D as a catalyst: application in phospholipid synthesis, molecular structure and protein engineering.J Biosci Bioeng. 2013 Sep;116(3):271-80. doi: 10.1016/j.jbiosc.2013.03.008. Epub 2013 Apr 29. J Biosci Bioeng. 2013. PMID: 23639419 Review.
Cited by
-
Phospholipase D: enzymology, functionality, and chemical modulation.Chem Rev. 2011 Oct 12;111(10):6064-119. doi: 10.1021/cr200296t. Epub 2011 Sep 22. Chem Rev. 2011. PMID: 21936578 Free PMC article. Review. No abstract available.
-
The catalytic and structural basis of archaeal glycerophospholipid biosynthesis.Extremophiles. 2022 Aug 17;26(3):29. doi: 10.1007/s00792-022-01277-w. Extremophiles. 2022. PMID: 35976526 Free PMC article. Review.
-
Human PLD structures enable drug design and characterization of isoenzyme selectivity.Nat Chem Biol. 2020 Apr;16(4):391-399. doi: 10.1038/s41589-019-0458-4. Epub 2020 Feb 10. Nat Chem Biol. 2020. PMID: 32042197
-
GDE7 produces cyclic phosphatidic acid in the ER lumen functioning as a lysophospholipid mediator.Commun Biol. 2023 May 16;6(1):524. doi: 10.1038/s42003-023-04900-4. Commun Biol. 2023. PMID: 37193762 Free PMC article.
References
-
- Bian, J. and Roberts, M.F. 1992. Comparison of surface properties and thermodynamic behavior of lyso- and diacylphosphatidylcholines. J. Colloid Interface Sci. 153: 420–428.
-
- Bruzik, K. and Tsai, M.D. 1984. Phospholipids chiral at phosphorus. Synthesis of chiral phosphatidylcholine and stereochemistry of phospholipase D. Biochemistry 23: 1656–1661. - PubMed
-
- Exton, J.H. 2002. Regulation of phospholipase D. FEBS Lett. 531: 58–61. - PubMed
-
- D'Arrigo, P., Piergianni, V., Scarcelli, D., and Servi, S. 1995. A spectrophotometric assay for phospholipase D. Anal. Chim. Acta 304: 249–254.
-
- Dittmer, J.C. and Lester, R.L. 1964. A simple, specific spray for the detection of phospholipids on thin-layer chromatograms. J. Lipid Res. 5: 126–127. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

