Novel phacB-encoded cytochrome P450 monooxygenase from Aspergillus nidulans with 3-hydroxyphenylacetate 6-hydroxylase and 3,4-dihydroxyphenylacetate 6-hydroxylase activities
- PMID: 17189487
- PMCID: PMC1828918
- DOI: 10.1128/EC.00226-06
Novel phacB-encoded cytochrome P450 monooxygenase from Aspergillus nidulans with 3-hydroxyphenylacetate 6-hydroxylase and 3,4-dihydroxyphenylacetate 6-hydroxylase activities
Abstract
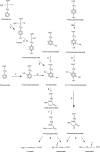
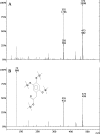
Aspergillus nidulans catabolizes phenylacetate (PhAc) and 3-hydroxy-, 4-hydroxy-, and 3,4-dihydroxyphenylacetate (3-OH-PhAc, 4-OH-PhAc, and 3,4-diOH-PhAc, respectively) through the 2,5-dihydroxyphenylacetate (homogentisic acid) catabolic pathway. Using cDNA subtraction techniques, we isolated a gene, denoted phacB, which is strongly induced by PhAc (and its hydroxyderivatives) and encodes a new cytochrome P450 (CYP450). A disrupted phacB strain (delta phacB) does not grow on 3-hydroxy-, 4-hydroxy-, or 3,4-dihydroxy-PhAc. High-performance liquid chromatography and gas chromatography-mass spectrum analyses of in vitro reactions using microsomes from wild-type and several A. nidulans mutant strains confirmed that the phacB-encoded CYP450 catalyzes 3-hydroxyphenylacetate and 3,4-dihydroxyphenylacetate 6-hydroxylations to generate 2,5-dihydroxyphenylacetate and 2,4,5-trihydroxyphenylacetate, respectively. Both of these compounds are used as substrates by homogentisate dioxygenase. This cytochrome P450 protein also uses PhAc as a substrate to generate 2-OH-PhAc with a very low efficiency. The phacB gene is the first member of a new CYP450 subfamily (CYP504B).
Figures




Similar articles
-
Disruption of phacA, an Aspergillus nidulans gene encoding a novel cytochrome P450 monooxygenase catalyzing phenylacetate 2-hydroxylation, results in penicillin overproduction.J Biol Chem. 1999 May 21;274(21):14545-50. doi: 10.1074/jbc.274.21.14545. J Biol Chem. 1999. PMID: 10329644
-
Purification and characterization of benzoate-para-hydroxylase, a cytochrome P450 (CYP53A1), from Aspergillus niger.Arch Biochem Biophys. 2001 Oct 15;394(2):245-54. doi: 10.1006/abbi.2001.2534. Arch Biochem Biophys. 2001. PMID: 11594739
-
Spectrophotometric determination of homogentisate using Aspergillus nidulans homogentisate dioxygenase.Anal Biochem. 1997 Feb 15;245(2):218-21. doi: 10.1006/abio.1996.9957. Anal Biochem. 1997. PMID: 9056215
-
Thermophilic cytochrome P450 enzymes.Biochem Biophys Res Commun. 2005 Dec 9;338(1):437-45. doi: 10.1016/j.bbrc.2005.08.093. Epub 2005 Aug 22. Biochem Biophys Res Commun. 2005. PMID: 16139791 Review.
-
Preparative use of isolated CYP102 monooxygenases -- a critical appraisal.J Biotechnol. 2006 Aug 5;124(4):662-9. doi: 10.1016/j.jbiotec.2006.02.013. Epub 2006 May 22. J Biotechnol. 2006. PMID: 16716428 Review.
Cited by
-
Whole-genome assembly and analysis of a medicinal fungus: Inonotus hispidus.Front Microbiol. 2022 Sep 6;13:967135. doi: 10.3389/fmicb.2022.967135. eCollection 2022. Front Microbiol. 2022. PMID: 36147857 Free PMC article.
-
New insights and advances on pyomelanin production: from microbial synthesis to applications.J Ind Microbiol Biotechnol. 2022 Jul 30;49(4):kuac013. doi: 10.1093/jimb/kuac013. J Ind Microbiol Biotechnol. 2022. PMID: 35482661 Free PMC article. Review.
-
Transport systems, intracellular traffic of intermediates and secretion of β-lactam antibiotics in fungi.Fungal Biol Biotechnol. 2020 Apr 25;7:6. doi: 10.1186/s40694-020-00096-y. eCollection 2020. Fungal Biol Biotechnol. 2020. PMID: 32351700 Free PMC article. Review.
-
Genome Assembly of the Fungus Cochliobolus miyabeanus, and Transcriptome Analysis during Early Stages of Infection on American Wildrice (Zizania palustris L.).PLoS One. 2016 Jun 2;11(6):e0154122. doi: 10.1371/journal.pone.0154122. eCollection 2016. PLoS One. 2016. PMID: 27253872 Free PMC article.
-
Resolving phenylalanine metabolism sheds light on natural synthesis of penicillin G in Penicillium chrysogenum.Eukaryot Cell. 2012 Feb;11(2):238-49. doi: 10.1128/EC.05285-11. Epub 2011 Dec 9. Eukaryot Cell. 2012. PMID: 22158714 Free PMC article.
References
-
- Arias-Barrau, E., A. Sandoval, G. Naharro, E. R. Olivera, and J. M. Luengo. 2005. A two-component hydroxylase involved in the assimilation of 3-hydroxyphenyl acetate in Pseudomonas putida. J. Biol. Chem. 280:26435-26447. - PubMed
-
- Cove, D. J. 1966. The induction and repression of nitrate reductase in the fungus Aspergillus nidulans. Biochim. Biophys. Acta 113:51-56. - PubMed
-
- Fernández-Cañón, J. M., B. Granadino, D. Beltrán-Balero de Bernabé, M. Renedo, E. Fernández-Ruiz, M. A. Peñalva, and S. R. de Córdoba. 1996. The molecular basis of alkaptonuria. Nat. Genet. 14:19-24. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

