Mad2 overexpression promotes aneuploidy and tumorigenesis in mice
- PMID: 17189715
- PMCID: PMC1850996
- DOI: 10.1016/j.ccr.2006.10.019
Mad2 overexpression promotes aneuploidy and tumorigenesis in mice
Abstract
Mad2 is an essential component of the spindle checkpoint that blocks activation of Separase and dissolution of sister chromatids until microtubule attachment to kinetochores is complete. We show here that overexpression of Mad2 in transgenic mice leads to a wide variety of neoplasias, appearance of broken chromosomes, anaphase bridges, and whole-chromosome gains and losses, as well as acceleration of myc-induced lymphomagenesis. Moreover, continued overexpression of Mad2 is not required for tumor maintenance, unlike the majority of oncogenes studied to date. These results demonstrate that transient Mad2 overexpression and chromosome instability can be an important stimulus in the initiation and progression of different cancer subtypes.
Figures
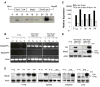

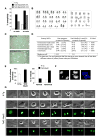

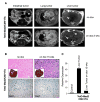


Comment in
-
Rb loss causes cancer by driving mitosis mad.Cancer Cell. 2007 Jan;11(1):1-3. doi: 10.1016/j.ccr.2006.12.006. Cancer Cell. 2007. PMID: 17222786 Review.
References
-
- Adams JM, Harris AW, Pinkert CA, Corcoran LM, Alexander WS, Cory S, Palmiter RD, Brinster RL. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature. 1985;318:533–538. - PubMed
-
- Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, Boldrick JC, Sabet H, Tran T, Yu X, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–511. - PubMed
-
- Baker DJ, Jeganathan KB, Cameron JD, Thompson M, Juneja S, Kopecka A, Kumar R, Jenkins RB, de Groen PC, Roche P, van Deursen JM. BubR1 insufficiency causes early onset of aging-associated phenotypes and infertility in mice. Nat Genet. 2004;36:744–749. - PubMed
-
- Bharadwaj R, Yu H. The spindle checkpoint, aneuploidy, and cancer. Oncogene. 2004;23:2016–2027. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

