Oxidized messenger RNA induces translation errors
- PMID: 17190801
- PMCID: PMC1765478
- DOI: 10.1073/pnas.0609737104
Oxidized messenger RNA induces translation errors
Abstract
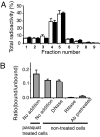
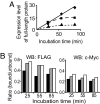
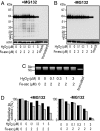
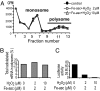
To investigate the effect of RNA oxidation on normal cellular functions, we studied the translation of nonoxidized and oxidized luciferase mRNA in both rabbit reticulocyte lysate and human HEK293 cells. When HEK293 cells transfected with nonoxidized mRNA encoding the firefly luciferase protein were cultured in the presence of paraquat, there was a paraquat concentration-dependent increase in the formation of luciferase short polypeptides (SPs) concomitant with an increase in 8-oxoguanosine. Short polypeptides were also formed when the mRNA was oxidized in vitro by the Fe-ascorbate-H(2)O(2) metal-catalyzed oxidation system before its transfection into cells. Translation of the in vitro oxidized mRNA in rabbit reticulocyte lysate also led to formation of SPs. The SPs formed by either procedure contained the N-terminal and the C-terminal portions of the tagged luciferase. In addition, the oxidized mRNA was able to associate with ribosomes to form polysomes similar to those formed with nonoxidized mRNA preparations, indicating that the oxidized mRNAs are mostly intact; however, their translation fidelity was significantly reduced. Nevertheless, our results indicate that the SPs were derived from both premature termination of the translation process of the oxidized mRNA and the proteolytic degradation of the modified full-length luciferase resulting from translation errors induced by oxidized mRNA. In light of these findings, the physiological consequences of mRNA oxidation are discussed.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Stadtman ER, Berlett BS. Chem Res Toxicol. 1997;10:485–494. - PubMed
-
- Beckman KB, Ames BN. Physiol Rev. 1998;78:547–584. - PubMed
-
- Evans MD, Dizdaroglu M, Cooke MS. Mutat Res. 2004;567:1–61. - PubMed
-
- Hofer T, Badouard C, Bajak E, Ravanat JL, Mattsson A, Cotgreave IA. Biol Chem. 2005;386:333–337. - PubMed
-
- Fiala ES, Conaway CC, Mathis JE. Cancer Res. 1989;49:5518–5522. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

