Structure and quantum chemical characterization of chloroperoxidase compound 0, a common reaction intermediate of diverse heme enzymes
- PMID: 17190816
- PMCID: PMC1765485
- DOI: 10.1073/pnas.0606285103
Structure and quantum chemical characterization of chloroperoxidase compound 0, a common reaction intermediate of diverse heme enzymes
Abstract
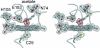
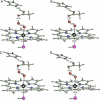
We have determined the crystal structure of the chloroperoxidase (CPO) hydroperoxo reaction intermediate (CPO compound 0) at 1.75-A resolution. The intermediate was generated through controlled photoreduction of the CPO oxygen complex during x-ray data collection, which was monitored by recording of the crystal absorption spectra. Initially, the peroxo-anion species was formed and then protonated to yield compound 0. Quantum chemical calculations indicate that the peroxo-anion species is not stable and collapses instantaneously to compound 0. Compound 0 is present in the ferric low-spin doublet ground state and is characterized by a long O O bond length of 1.5 A and a Fe O bond distance of 1.8 A, which is also observed in the crystal structure.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

