The CobraPLA during anesthesia with controlled ventilation: a clinical trial of efficacy
- PMID: 17191308
- PMCID: PMC2687819
- DOI: 10.3349/ymj.2006.47.6.799
The CobraPLA during anesthesia with controlled ventilation: a clinical trial of efficacy
Abstract
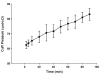
The CobraPLA (CPLA) is a relatively new supraglottic airway device that has not been sufficiently investigated. Here, we performed a prospective observational study to evaluate the efficacy of the CPLA during controlled ventilation. In 50 anesthetized and paralyzed patients undergoing elective surgery a CPLA was inserted and inflated to an intracuff pressure of 60 cm H2O. The success rate of insertion upon the first attempt was 82% (41/50), with a mean insertion time of 16.3 +/- 4.5 seconds. The adequacy of ventilation was assessed by observing the end tidal CO2 waveform, movement of the chest wall, peak airway pressure (13.5 cm H2O), and leak fraction (4%). We documented the airway sealing pressure (22.5 cm H2O) and noted that the the site of gas leaks at that pressure were either at the neck (52%), the abdomen (46%), or both (2%). In 44 (88%) patients, the vocal cords were visible in the fiberoptic view through the CPLA. There was no gastric insufflation during the anesthesia. Respiratory and hemodynamic parameters remained stable during CPLA insertion. Postoperative blood staining of CPLA was minimal, occurring in 22% (11/50) of patients. Mild and moderate throat soreness was reported in 44% (22/50) and 4% (2/50) of patients, respectively. Lastly, mild dysphonia was observed in 6% (3/50) of patients and mild dysphagia in 10% (5/50) of patients. Our results indicated that the CPLA is both easy to place and allows adequate ventilation during controlled ventilation.
Figures
Similar articles
-
Insertion characteristics, sealing pressure and fiberoptic positioning of CobraPLA in children.Paediatr Anaesth. 2007 Oct;17(10):977-82. doi: 10.1111/j.1460-9592.2007.02241.x. Paediatr Anaesth. 2007. PMID: 17767635
-
A prospective, randomized comparison of cobra perilaryngeal airway and laryngeal mask airway unique in pediatric patients.Anesth Analg. 2008 Nov;107(5):1523-30. doi: 10.1213/ane.0b013e3181852617. Anesth Analg. 2008. PMID: 18931209 Clinical Trial.
-
The PAxpress is an effective ventilatory device but has an 18% failure rate for flexible lightwand-guided tracheal intubation in anesthetized paralyzed patients.Can J Anaesth. 2003 May;50(5):495-500. doi: 10.1007/BF03021063. Can J Anaesth. 2003. PMID: 12734160 Clinical Trial. English, French.
-
The new perilaryngeal airway (CobraPLA) is as efficient as the laryngeal mask airway (LMA) but provides better airway sealing pressures.Anesth Analg. 2004 Jul;99(1):272-278. doi: 10.1213/01.ANE.0000117003.60213.E9. Anesth Analg. 2004. PMID: 15281543 Free PMC article. Clinical Trial.
-
An evaluation of the insertion and function of a new supraglottic airway device, the King LT, during spontaneous ventilation.Anesth Analg. 2006 Feb;102(2):621-5. doi: 10.1213/01.ane.0000189101.26403.06. Anesth Analg. 2006. PMID: 16428573
References
-
- Brain AI. The laryngeal mask - a new concept in airway management. Br J Anaesth. 1983;55:801–805. - PubMed
-
- Brain AI. Three cases of difficult intubation overcome by the laryngeal mask airway. Anaesthesia. 1985;40:353–355. - PubMed
-
- Frass M, Frenzer R, Rauscha F, Weber H, Pacher R, Leithner C. Evaluation of esophageal tracheal combitube in cardiopulmonary resuscitation. Crit Care Med. 1987;15:609–611. - PubMed
-
- Devitt JH, Wenstone R, Noel AG, O'Donnell MP. The laryngeal mask airway and positive-pressure ventilation. Anesthesiology. 1994;80:550–555. - PubMed
-
- Cook TM, McKinstry C, Hardy R, Twigg S. Randomized crossover comparison of the ProSeal™ laryngeal mask airway with the Laryngeal Tube® during anaesthesia with controlled ventilation. Br J Anaesth. 2003;91:678–683. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical