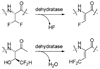
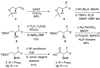
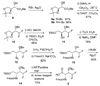
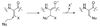Efficient synthesis of suitably protected beta-difluoroalanine and gamma-difluorothreonine from L-ascorbic acid
- PMID: 17192080
- PMCID: PMC2593874
- DOI: 10.1021/ol062401a
Efficient synthesis of suitably protected beta-difluoroalanine and gamma-difluorothreonine from L-ascorbic acid
Abstract
[reaction: see text] Fluorinated amino acids are useful building blocks for the preparation of biologically active peptides and peptidomimetics with increased metabolic stability. We report here the synthesis of two fluorinated amino acids, beta-difluoroalanine and gamma-difluorothreonine, as analogues of Ser and Thr, respectively. These compounds were suitably protected for Fmoc-based solid-phase peptide synthesis. Once incorporated into peptides, they may serve as alternative substrates or inhibitors of lantibiotic synthetases that posttranslationally dehydrate Ser and Thr residues to dehydroalanine and dehydrobutyrine, respectively.
Similar articles
-
Using expressed protein ligation to probe the substrate specificity of lantibiotic synthetases.Methods Enzymol. 2009;462:117-34. doi: 10.1016/S0076-6879(09)62006-1. Methods Enzymol. 2009. PMID: 19632472
-
Stereoselective synthesis of anti-alpha-(difluoromethyl)-beta-amino alcohols by boronic acid based three-component condensation. Stereoselective preparation of (2S,3R)-difluorothreonine.J Org Chem. 2002 May 31;67(11):3718-23. doi: 10.1021/jo011116w. J Org Chem. 2002. PMID: 12027685
-
A concise methodology for the stereoselective synthesis of O-glycosylated amino acid building blocks: complete 1H NMR assignments and their application in solid-phase glycopeptide synthesis.J Pept Res. 1998 Sep;52(3):165-79. doi: 10.1111/j.1399-3011.1998.tb01473.x. J Pept Res. 1998. PMID: 9774229
-
Fluoro amino acids: a rarity in nature, yet a prospect for protein engineering.Biotechnol J. 2015 Mar;10(3):427-46. doi: 10.1002/biot.201400587. Epub 2015 Mar 2. Biotechnol J. 2015. PMID: 25728393 Review.
-
Recent advances in the synthesis of fluorinated amino acids and peptides.Chem Commun (Camb). 2023 Feb 2;59(11):1434-1448. doi: 10.1039/d2cc06787k. Chem Commun (Camb). 2023. PMID: 36651307 Review.
Cited by
-
Flow microreactor synthesis in organo-fluorine chemistry.Beilstein J Org Chem. 2013 Dec 5;9:2793-802. doi: 10.3762/bjoc.9.314. Beilstein J Org Chem. 2013. PMID: 24367443 Free PMC article. Review.
-
Asymmetric α-Fluoroalkyl-α-Amino Acids: Recent Advances in Their Synthesis and Applications.Molecules. 2024 Mar 21;29(6):1408. doi: 10.3390/molecules29061408. Molecules. 2024. PMID: 38543045 Free PMC article. Review.
-
Synthesis and Deployment of an Elusive Fluorovinyl Cation Equivalent: Access to Quaternary α-(1'-Fluoro)vinyl Amino Acids as Potential PLP Enzyme Inactivators.J Am Chem Soc. 2017 Oct 11;139(40):14077-14089. doi: 10.1021/jacs.7b04690. Epub 2017 Sep 28. J Am Chem Soc. 2017. PMID: 28906111 Free PMC article.
-
Novel synthesis of pseudopeptides bearing a difluoromethyl group by Ugi reaction and desulfanylation.Beilstein J Org Chem. 2011;7:1070-4. doi: 10.3762/bjoc.7.123. Epub 2011 Aug 8. Beilstein J Org Chem. 2011. PMID: 21915210 Free PMC article.
References
-
- Walsh C. Tetrahedron. 1982;38:871.
- Walsh CT. Annu. Rev. Biochem. 1984;53:493. - PubMed
- Silverman RB. Mechanism-based Enzyme Inactivation: Chemistry and Enzymology. Boca Raton, FL: CRC Press; 1988.
-
- Botes D, Tuinman A, Wessels P, Viljoen C, Kruger H, Williams DH, Santikarn S, Smith R, Hammond SJ. Chem. Soc. Perkin Trans. 1984;1:2311.
- Painuly P, Perez R, Fukai T, Shimizu Y. Tetrahedron Lett. 1988;29:11.
- Dawson RM. Toxicon. 1998;36:953. - PubMed
-
- Namikoshi M, Rinehart KL. J. Industr. Microbiol. 1996;17:373.
- Botes D, Wessels P, Kruger H, Runnegar MTC, Santikarn S, Smith R, Barna JCJ, Williams DH. J. Chem. Soc. Perkin Trans. 1985;1:2747.
- Rinehart KL, Harada K, Namikoshi M, Chen C, Harvis C, Munro MHG, Blunt J, Mulligan P, Beasley V, Dahlem A, Carmicheal W. J. Am. Chem. Soc. 1988;110:8557.
-
- Chatterjee C, Paul M, van der Donk WA. Chem. Rev. 2005;105:633. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical