Two complex, adenovirus-based vaccines that together induce immune responses to all four dengue virus serotypes
- PMID: 17192403
- PMCID: PMC1797786
- DOI: 10.1128/CVI.00330-06
Two complex, adenovirus-based vaccines that together induce immune responses to all four dengue virus serotypes
Abstract
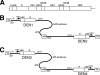
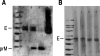
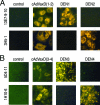
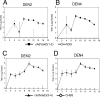
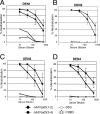
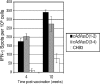
Dengue virus infections can cause hemorrhagic fever, shock, encephalitis, and even death. Worldwide, approximately 2.5 billion people live in dengue-infested regions with about 100 million new cases each year, although many of these infections are believed to be silent. There are four antigenically distinct serotypes of dengue virus; thus, immunity from one serotype will not cross-protect from infection with the other three. The difficulties that hamper vaccine development include requirements of the natural conformation of the envelope glycoprotein to induce neutralizing immune responses and the necessity of presenting antigens of all four serotypes. Currently, the only way to meet these requirements is to use a mixture of four serotypes of live attenuated dengue viruses, but safety remains a major problem. In this study, we have developed the basis for a tetravalent dengue vaccine using a novel complex adenovirus platform that is capable of expressing multiple antigens de novo. This dengue vaccine is constructed as a pair of vectors that each expresses the premembrane and envelope genes of two different dengue virus serotypes. Upon vaccination, the vaccine expressed high levels of the dengue virus antigens in cells to mimic a natural infection and induced both humoral and cellular immune responses against multiple serotypes of dengue virus in an animal model. Further analyses show the humoral responses were indeed neutralizing against all four serotypes. Our studies demonstrate the concept of mimicking infections to induce immune responses by synthesizing dengue virus membrane antigens de novo and the feasibility of developing an effective tetravalent dengue vaccine by vector-mediated expression of glycoproteins of the four serotypes.
Figures
Similar articles
-
An adenovirus type 5 (AdV5) vector encoding an envelope domain III-based tetravalent antigen elicits immune responses against all four dengue viruses in the presence of prior AdV5 immunity.Vaccine. 2009 Oct 9;27(43):6011-21. doi: 10.1016/j.vaccine.2009.07.073. Epub 2009 Aug 7. Vaccine. 2009. PMID: 19665609
-
Induction of bivalent immune responses by expression of dengue virus type 1 and type 2 antigens from a single complex adenoviral vector.Am J Trop Med Hyg. 2007 Apr;76(4):743-51. Am J Trop Med Hyg. 2007. PMID: 17426182
-
An adenovirus prime/plasmid boost strategy for induction of equipotent immune responses to two dengue virus serotypes.BMC Biotechnol. 2007 Feb 15;7:10. doi: 10.1186/1472-6750-7-10. BMC Biotechnol. 2007. PMID: 17302980 Free PMC article.
-
Challenges for the formulation of a universal vaccine against dengue.Exp Biol Med (Maywood). 2013 May;238(5):566-78. doi: 10.1177/1535370212473703. Exp Biol Med (Maywood). 2013. PMID: 23856907 Review.
-
Recent progress in dengue vaccine research and development.Curr Opin Mol Ther. 2010 Feb;12(1):31-8. Curr Opin Mol Ther. 2010. PMID: 20140814 Review.
Cited by
-
Next-generation dengue vaccines: novel strategies currently under development.Viruses. 2011 Oct;3(10):1800-14. doi: 10.3390/v3101800. Epub 2011 Sep 26. Viruses. 2011. PMID: 22069516 Free PMC article.
-
Experimental vaccines against potentially pandemic and highly pathogenic avian influenza viruses.Future Virol. 2013 Jan 1;8(1):25-41. doi: 10.2217/fvl.12.122. Future Virol. 2013. PMID: 23440999 Free PMC article.
-
Induction of neutralizing antibody response against four dengue viruses in mice by intramuscular electroporation of tetravalent DNA vaccines.PLoS One. 2014 Jun 2;9(6):e92643. doi: 10.1371/journal.pone.0092643. eCollection 2014. PLoS One. 2014. PMID: 24887426 Free PMC article.
-
A tetravalent dengue vaccine based on a complex adenovirus vector provides significant protection in rhesus monkeys against all four serotypes of dengue virus.J Virol. 2008 Jul;82(14):6927-34. doi: 10.1128/JVI.02724-07. Epub 2008 May 14. J Virol. 2008. PMID: 18480438 Free PMC article.
-
Computational analysis and identification of amino acid sites in dengue E proteins relevant to development of diagnostics and vaccines.Virus Genes. 2007 Oct;35(2):175-86. doi: 10.1007/s11262-007-0103-2. Epub 2007 May 17. Virus Genes. 2007. PMID: 17508277
References
-
- Barouch, D. H., and G. J. Nabel. 2005. Adenovirus vector-based vaccines for human immunodeficiency virus type 1. Hum. Gene Ther. 16:149-156. - PubMed
-
- Blaney, J. E., Jr., J. M. Matro, B. R. Murphy, and S. S. Whitehead. 2005. Recombinant, live-attenuated tetravalent dengue virus vaccine formulations induce a balanced, broad, and protective neutralizing antibody response against each of the four serotypes in rhesus monkeys. J. Virol. 79:5516-5528. - PMC - PubMed
-
- Chambers, T. J., Y. Liang, D. A. Droll, J. J. Schlesinger, A. D. Davidson, P. J. Wright, and X. Jiang. 2003. Yellow fever virus/dengue-2 virus and yellow fever virus/dengue-4 virus chimeras: biological characterization, immunogenicity, and protection against dengue encephalitis in the mouse model. J. Virol. 77:3655-3668. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

