Identification of an N-acetylglucosamine transporter that mediates hyphal induction in Candida albicans
- PMID: 17192409
- PMCID: PMC1805087
- DOI: 10.1091/mbc.e06-10-0931
Identification of an N-acetylglucosamine transporter that mediates hyphal induction in Candida albicans
Abstract
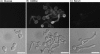
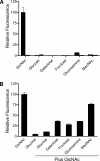
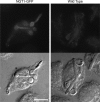
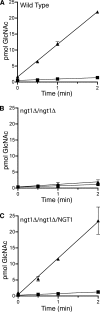
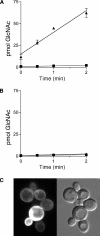
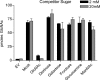
The sugar N-acetylglucosamine (GlcNAc) plays an important role in nutrient sensing and cellular regulation in a wide range of organisms from bacteria to humans. In the fungal pathogen Candida albicans, GlcNAc induces a morphological transition from budding to hyphal growth. Proteomic comparison of plasma membrane proteins from buds and from hyphae induced by GlcNAc identified a novel hyphal protein (Ngt1) with similarity to the major facilitator superfamily of transporters. An Ngt1-GFP fusion was detected in the plasma membrane after induction with GlcNAc, but not other related sugars. Ngt1-GFP was also induced by macrophage phagocytosis, suggesting a role for the GlcNAc response in signaling entry into phagolysosomes. NGT1 is needed for efficient GlcNAc uptake and for the ability to induce hyphae at low GlcNAc concentrations. High concentrations of GlcNAc could bypass the need for NGT1 to induce hyphae, indicating that elevated intracellular levels of GlcNAc induce hyphal formation. Expression of NGT1 in Saccharomyces cerevisiae promoted GlcNAc uptake, indicating that Ngt1 acts directly as a GlcNAc transporter. Transport mediated by Ngt1 was specific, as other sugars could not compete for the uptake of GlcNAc. Thus, Ngt1 represents the first eukaryotic GlcNAc transporter to be discovered. The presence of NGT1 homologues in the genome sequences of a wide range of eukaryotes from yeast to mammals suggests that they may also function in the cellular processes regulated by GlcNAc, including those that underlie important diseases such as cancer and diabetes.
Figures


References
-
- Abramson J., Iwata S., Kaback H. R. Lactose permease as a paradigm for membrane transport proteins. Mol. Membr. Biol. 2004;21:227–236. - PubMed
-
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. - PubMed
-
- Bailey T., Elkan C. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proceedings of the Second International Conference on Intelligent Systems for Molecular Biology; Menlo Park, CA: AAAI Press; 1994. pp. 28–36. - PubMed
-
- Berman J., Sudbery P. E. Candida albicans: a molecular revolution built on lessons from budding yeast. Nat. Rev. Genet. 2002;3:918–930. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

