Bidirectional activity-dependent regulation of neuronal ion channel phosphorylation
- PMID: 17192433
- PMCID: PMC6674719
- DOI: 10.1523/JNEUROSCI.3970-06.2006
Bidirectional activity-dependent regulation of neuronal ion channel phosphorylation
Abstract
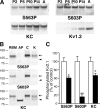
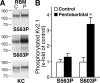
Activity-dependent dephosphorylation of neuronal Kv2.1 channels yields hyperpolarizing shifts in their voltage-dependent activation and homoeostatic suppression of neuronal excitability. We recently identified 16 phosphorylation sites that modulate Kv2.1 function. Here, we show that in mammalian neurons, compared with other regulated sites, such as serine (S)563, phosphorylation at S603 is supersensitive to calcineurin-mediated dephosphorylation in response to kainate-induced seizures in vivo, and brief glutamate stimulation of cultured hippocampal neurons. In vitro calcineurin digestion shows that supersensitivity of S603 dephosphorylation is an inherent property of Kv2.1. Conversely, suppression of neuronal activity by anesthetic in vivo causes hyperphosphorylation at S603 but not S563. Distinct regulation of individual phosphorylation sites allows for graded and bidirectional homeostatic regulation of Kv2.1 function. S603 phosphorylation represents a sensitive bidirectional biosensor of neuronal activity.
Figures






Comment in
-
Ps in the (channel) pod are not alike..Epilepsy Curr. 2007 Sep-Oct;7(5):136-7. doi: 10.1111/j.1535-7511.2007.00203.x. Epilepsy Curr. 2007. PMID: 17998975 Free PMC article. No abstract available.
References
-
- Antonucci DE, Lim ST, Vassanelli S, Trimmer JS. Dynamic localization and clustering of dendritic Kv2.1 voltage-dependent potassium channels in developing hippocampal neurons. Neuroscience. 2001;108:69–81. - PubMed
-
- Colbert CM, Pan E. Ion channel properties underlying axonal action potential initiation in pyramidal neurons. Nat Neurosci. 2002;5:533–538. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
